|
|
|
Does colony size affect embryo abundance? Study of the bryozoan, Bugula neritna.
|
|
Callum Lister 2015
|
|
|
|
Summary | |
B ryozoans are both marine and freshwater sessile invertebrates, which are easily mistaken for algae and coral. There are currently 4000 extant species of bryozoa, though it is likely that there is currently double that number in existence (Ryland 2005). The species Bugula neritina is a cheilostome bryozoan which is an invasive cryptic species with a cosmopolitan distribution and has been well studied both in the field and laboratory. B. neritina colonies are arborescent, reaching a height of about 80mm and is purplish-brown in colour. The budding individuals which make up the colony are called zooids which have only a light calcium carbonate exoskeleton.
Ecologically, they are an important group, as it is a major component of marine biofouling; dominating anthropogenic surfaces and highly polluted areas. B. neritina are valued for their potential medical treatments of cancer and Alzheimer's disease as it produces bryostatins. They are also important evolutionarily as bryozoans are a long-living phylum that has a good fossil record.
|
 |
| Figure 1 |
|
|
|
Physical Description | |
B. neritina form upright branching colonies which are purplish-brown or can be a dark reddish-purple in colour which turns into a translucent brown when preserved, and reach a maximum height of approximately 80mm (Pearse et al., 1987; Winston 1977; Yu et al., 2007). The colony consists of individual zooids which measured an average of 0.95 x 0.26mm and proximally narrow. Each zooid is made up of a cystid which is the zooecium and body wall, and polypide which is the feeding organ. Each branch of the colony is made up of two staggered rows of cystids. Avicularia and spines are absent in B. neritina, however, the distal margin forms an angular projection (denticles) (Ryland 2005). In B. neritina, the zooid cystid is lightly calcified, giving thecolony a horny/rough texture (Ryland 2005). The feeding organ or lophophore, has on average 20-24 translucent, crown shaped tentacles which are lined with cilia (Ruppert et al., 2004). When disturbed, zooids will retract their lophophore. The digestive tract has several regions, finishing at the anus which is located outside of the tentacles (Ectoprocta translates to “outside anus”) (Ryland 2005).
Embryos which are a dark down colour are externally brooded in the ovicell which were measured to have a diameter of approximately 0.25mm. The ovicell are spherical structures attached to the distal end of the zooid which have the appearance of small beads lining the branches (Figure 1).
|
 |
| Figure 2 |
|
|
|
Ecology | |
B.neritina is an invasive species and a major competitor in the fouling community (Sutherland & Karlson, 1977). As mentioned in Biogeographic Distribution; B. neritina is one of the main organisms to encrust and foul man-made structures and ships, however, this species is also able to foul other organisms including; seagrasses, oysters, ascidians, and other bryozoans (Keough 1999; Ryland et al., 2011). The increase of tolerance and resistance to toxicants has enabled it to survive and grow successfully in highly polluted environments and gives it a competitive advantage over more highly sensitive species (Piola & Johnston, 2006). As well as dominating polluted areas, B. neritina are able to tolerate relatively large fluctuations in environmental parameters (Table1). B. neritina has a cosmopolitan distribution despite the larvae’s low dispersal potential (Keough & Chernoff, 1987), this reflects the species success as a fouling organism and also dispersal through anthropogenic transport (Carlton & Geller 1993; Mcgovern & Hellberg, 2003).
Table 1: Tolerance levels of Environmental parameters for the bryozoan, Bugula neritina.
|
Environmental Parameter
|
Minimum
|
Maximum
|
|
Temperature (oC)
|
2.2
|
30
|
|
Salinity (‰)
|
18
|
40
|
|
pH
|
Unknown
|
Unknown
|
|
Dissolved Oxygen
|
Unknown
|
Unknown
|
Grazing organisms such as sea urchins and pomacentrid fish are the main predators of adult B. neritina. Nudibranchs have also been recorded feeding upon bryozoans including B. neritina (Avila 1995; Carte & Faulkner, 1986; Miller 1961). However, B. neritina palatability to predators varies geographically (Mcgovern & Hellberg, 2003). In North Carolina, B. neritina larvae and adults are unpalatable and chemically defended to protect against potential predators such as certain fish and crab species (Lindquist & Hay, 1996; Mcgovern & Hellberg, 2003). Whereas, in Delaware, B. neritina larvae are predated on by the same fish species that avoid larvae in North Carolina (Mcgovern &Hellberg, 2003). Sponges, algae, ascidians and other biofouling organisms are also in direct competition with bryozoans for food and space.
This species has a complexity of different haplotypes which can only be distinguished using molecular analysis. Studies have shown that there are at least three different haplotypes. They have been designated as 'Type S' which is shallow-water and cosmopolitan; ‘TypeD’ is open water; and the North Atlantic ‘Type N’ (Davidson & Haygood 1999; Fehlman-Ale et al., 2014; McGovern & Hellberg 2003; Mackie et al. 2006). However, Fehlman-Ale and colleagues (2014) also found haplotype ‘N’ occurring in Central California and north-eastern Australia.
B. neritina colonies are the source of bryostatin, a chemical compound, which has been shown to be effective anticancer compound (Davidson & Haygood, 1999). The source of the bryostatins is a species of bacterium, Candidatus Endobugula sertula, which is symbiotic with haplotype ‘D' (Davidson & Haygood, 1999; Davidson et al., 2001).
|
|
|
Life History and Behaviour |
Reproduction | |
Ectoprocts reproduce using two different methods; asexually by budding and sexually by spermcasting. Spermcast mating is the act of dispersing aquatic spermatozoa to fertilize eggs which have been retained by the individual (Bishop & Pemberton, 2006). B. neritina are hermaphroditic, with individual zooids producing both eggs and sperm (Ruppert et al., 2004).
The testes are located at the basal end of the funiculus. The gonads are temporary, and gametes are firstly discharged into the metacoel, before migrating to the mesocoel. As they don’t have gonoducts, sperm are released from the coelom through the terminal pores at the tips of the tentacles, with fertilisation being either internal (sperm entering through the intertentacular organ) or external (adhere to the tentacles of other zooids) (Ruppert et al., 2004). Before being released to an external brooding area, the eggs travel along a ciliated groove in the mesothelial through the intertentacular organ to the coelomopore - an opening at the base of the two dorsalmost tentacles. Each zooid produces a single embryo at a time, which is brooded in the ovicell; a large, white, spherical structure attached to the distal end of the zooid (Ruppert et al., 2004) which are oriented at angles to the axis of the branch.
Zooids release eggs around the middle of their lifespan and don't release sperm until near the end (protandry), thus preventing self-fertilization (Ryland 1976). When released from the ovicell, often around dawn, the larvae are lecithotrophic which have neither mouths nor digestive tracts and rely on the yolk sac for nutrients. Sexual maturity is reached at 6-8 weeks.
|
|
|
Embryo Abundance Experiment | |
The fitness of any individual is largely dependent on the number of viable offspring it produces. For that reason, the amount of investment put into reproduction is an important characteristic of the life history of an individual and, therefore, species as a whole (Jackson & Wertheimer, 1985). There is a lack of quantitative information on embryo production by brooding species of bryozoans. From observations, embryos tend to be concentrated in the more central, older part of a colony, these observations formed the bases of the hypothesis that embryo density increases with increasing colony size. This study aims to investigate the relationship between abundance of embryos and colony size in the bryozoan, B. neritina.
Methods
Settlement plates were left submerged at Many Harbour, Brisbane, Queensland, for approximately 4 weeks from the end of March into April, before being collected. They were then taken back to the aquarium at the University of Queensland to be kept when not being counted.
From the plates, 32 B. neritina colonies were picked at random. Carefully using surgical tweezers, anything fouling B. neritina (i.e. algae, detritus and snails) were removed. Total embryo counts were then done for each colony using a dissecting microscope. The size of each colony was based off its density which was determined using displacement methods. After being counted, each colony was then rigorously yet cautiously flicked (as not to break the colony apart) 5 times, then using paper towel was pressed dried for 5 seconds. Once drying was complete, the colony was placed into a 15ml tube which had been filled with 5ml of water, using the measuring markings on the side of the tube water displacement and, therefore, density was recorded to the nearest 0.1ml.
The effect of colony size on embryo abundance was tested using regression analysis. Statistical analysis was done using Rstudio, significance level was set at α = 0.05.
Result
Size of the colony explained 50% of the variation in embryo abundance. There is a positive linear relationship between the two variables, as the number of eggs increased with increasing colony size (Figure 2) (equation: number off eggs = -161.4 + 2440 x colony size, F1,30=30.56, p= 5.251e-6, r2=0.5).
Discussion
These findings, although emerging from a limited experimental design due to the logistical limitations, illustrated that there is a significant relationship between size of the colony and embryo abundance (Figure 2). There was a positive correlation between number of embryos present in an individual colony and colony size. Each of the 32 colonies counted had embryos present. However,embryos were not randomly distributed within each of the colonies, but rathershowing a density zonation pattern with distance from colony growing tips. This is supported by a study conducted by Jackson & Wertheimer (1985). This distribution would be one explanation for the large colonies having more embryos; as the branches on smaller younger colonies would have minimal distance from growing tips and, therefore, not being able to support high embryo density due to zonation. Whereas, compared to larger colonies, distance from growing tips would be greater and able to support an increase in embryo density. Additionally in larger, more mature colonies, there were fewer growing tips, and presumably a reduction in growth. Therefore, less energy is invested in growth in largecolonies, allowing more energy to be spent on reproduction (Keough 1989).
This study used colony density as a way of determining size, however, using the displacement method was not a true indication to size, as some colonies may have had more smaller branches, whereas, others may have been longer with less branches. Also as density intervals were 0.1ml which might have been too great to show smaller changes in size. This study was only able to use B. neritina colonies from one location and wasn’t able to measure the changes in embryo abundance during different seasons, therefore, from these results it is not possible to generalise for all B. neritina populations or different species of bryozoan. Future studies look at differences in embryo density in different locations and also with frequent sampling across the year to monitor seasonal differences. The model only explained 50% of the embryo abundance variation, therefore, future research should also study biotic and abiotic variables such as water temperature, pH levels, water flow and predator abundance and how they impact embryo abundance.
|
 |
| Figure 3 |
|
|
|
Larvae Locomotion and Behaviour | |
Locomotion is achieved by coordination of ciliated coronal cells (Figure 3) that cover most of the outer surface of the larva (Woollacott & Zimmer, 1971). Larvae are initially photopositive; many have pigment spots which may be light sensitive (Burgess et al., 2009). Shortly later - usually within a few hours of release - becoming negatively phototaxic and geopositive, swimming to the bottom and settling (Burgess et al., 2009). Once on the bottom, specific chemical, physical and biological cues are used to determine suitability of the area for the adult, the potential substrate is probed using the pyriform organ which is a collection of extended ciliary bundles (Woollacott & Zimmer, 1971; Yu et al., 2007). B. neritina larvae depend on the interaction of water flow and substratum type (Walters et al., 1999). Walters and colleagues (1999) found that B. neritina larvae were most likely to swim away and, therefore, reject a still water area than an area in high flow conditions, which would be advantageous assuming food availability is greatest in areas with high flow. Larval settlement is also influenced by the presence of biofilms on the substrate (Dahms et al., 2004). Biofilms are microorganisms such as bacteria and diatoms, bound together to create a film of extracellular polymeric substances (Zardus et al., 2008). Dahms and colleagues (2004) found that the presence of Nitzschia frustulum biofilm resulted the lowest settlement of B. neritina larvae.Other endogenous factors such as larval age, physiological condition, size and genotype can affect settlement decisions. Once a suitable site has been located, permanent attachment takes place.
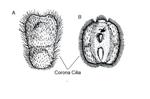
Attachment results in the release of adhesive cement which is initiated by the eversion of the metasomal sac (Loeb & Walker, 1977). At that point, the metamorphosed larva becomes an ancestrula, beginning a new colony. Larvae typically settle throughout the year, although settlement is reduced during midwinter (Sutherland & Karlson, 1977).
|
 |
| Figure 4 |
|
|
|
Development | |
Cleavage of the embryo is radial, holoblastic and nearly equal, creating a coeloblastula. Two layers of cells, the upper animal plate and the lower vegetal plate are produced by early cleavage. Gymnolaemates undergo gastrulation, which occurs when division of four blastomeres in the vegetal plate contribute daughter blastomeres to the blastocoel which become the endoderm and mesoderm (Ruppert et al., 2004).
Lecithotrophic larvae after attaching metamorphose into an ancestrula (Figure 4), each colony begins from this sexually produced zooid. This zooid undergoes asexual budding to produce a pair of daughter cells, which themselves form buds, the size of the colony increases with continuous asexual reproduction (Ruppert et al., 2004). The new daughter zooids start out as the cystid,from which the ectoderm and mesothelium develops into a new polypide (Ruppert et al., 2004). As Bugula are arborescent, colony growth only occurs at tips of each branch. Although each zooid in the colony are genetically identical,differences in phenotype (polymorphism) occur, with zooids being different in size or shape.
Field studies of B. neritina from different habitats in Australia and North America show significant variation in life history, due to genetic or early environmental effects (Keough, 1989). Depending on location, life history of B. neritina may include a period of dormancy, inwhich the colony recedes back to a regenerative holdfast (Dyrynda & Ryland, 1982). This senescence occurs at differing times of year dependent upon water temperature (Keough & Chernoff, 1987).
|
 |
| Figure 5 |
|
|
|
|
Anatomy and Physiology |
Colony and Zooid Structure | |
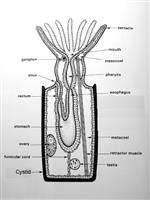
In bryozoans, colonies are made up of individual zooids, however, the whole colony to an extent, appears to be coordinated through physiology and behaviour (Ryland 2005). Ancestrula undergoes asexual budding, in which daughter zooids are formed. In a mature adult colony, the outer tips of the branches are the newest budding zooids while the base near the ancestrula contain the older zooids. The ultimate shape and structure of the colony is governed by the pattern in which daughter zooids bud from the ancestrula (Ryland 2005). Bugula neritina colonies form erect, fruticose structures which resemble seaweed. Each branch of the colony is made up of two staggered rows of zooecia (Figure 5) (Ruppert et al., 2004). The rhizoids (kenozooids) which are the holdfast strains, grow away from the colony at the base. Autozooids are the fully developed feeding zooids; are able to bud from the kenozooids after a senescence or disturbance (Keough & Chernoff, 1987). Colonies are comprised primarily by these autozooids, though through polymorphism there are several other type of zooids called heterozooids which are non-feeding (polypide is reduced or absent), and sterile (Ruppert et al., 2004). Three types of heterozooid are; avicularia, vibracula and kenozooids.
Avicularia occur in cheilostomata, they are defensive zooids which prevents larvae and other organisms from settling on the colony (Ruppert et al., 2004). However, unlike other species of Bugula, B. neritina does not have anavicularia. Vibracula similar to avicularia occur in some cheilostomes and serves to prevent larvae and debris from settling on the colony (Ryland 2005). In vibracula the cystid is reduced and the operculum is modified into a long bristle or seta which is freely movable (Ruppert et al., 2004). Kenozooids consist of body wall enclosing tissue strands and constitute stalks, rhizoids and holdfasts which attach to the substrate (Ruppert et al., 2004).
Pore plates in the dividing wall allow the funicular system (see Excretory, Circulatory and Respiratory Systems) to transport nutrients and communicate between neighbouring non-feeding zooids (ovicells or gonozooids) or to areas such as the growing edge (Bobin 1977). Though the colony can be partially destroyed and will not cause harm to the remaining part of the colony (Rylan 2005).
|
 |
| Figure 6 |
|
|
|
Feeding and Digestive Systems | |
Bryozoans are suspension feeders (Ruppert et al., 2004). Their feeding apparatus consists of a ring of extended ciliated tentacles which form a tentacular crown or lophophore (Figure 6), with its mouth at the centre of the base (Riisgard & Manriquez, 1997). When the lophophore is protruded, the cilia creates a current which draw microscopic plankton and organic particles down towards their mouth (Pratt 2004). In larger colonies, individual zooids work together to create a stronger current (Pratt, 2004). Particle rejection is not well understood, they may use serval methods to accomplish this; tentacle flicking, closing of the mouth, funnel closure and or being passed between tentacles (Ruppert et al., 2004).
In marine ectoprocts the number of tentacles can vary between 8 and 40, depending on nutrition (Jebram 1979), young zooids also tend to have smaller and fewer tentacles (Gordon 1974).
The lophophore leads into a muscular pharynx which acts as a suction-pump, then into a tubular esophagus, before finally ending in the U-shaped stomach. The stomach is comprised of three sections: the anterior tubular cardia, the medial sac-like caecum, and the posterior tubular pyloris (Ryland 1976; Ruppert et al., 2004).
|
 |
| Figure 7 |
|
|
|
Excretory, Circulatory and Respiratory Systems | |
B. neritina lack anephridia - a waste discharging tubule with an external excretory pore (Ruppert et al., 2004). Waste materials are passed into the intestine from the pylorus and ultimately exits through the anus, located near the base of the mostdorsal pair of tentacles (Ryland 1976).
As bryozoans lack specialised respiratory organs (i.e. gills), gas exchange and nitrogen excretion (ammonia) are accomplished over exposed body surfaces, especially the epithelium of the lophophore. There is also no hemal system (i.e. no heart or blood vessels). The transport of gasses and wastes is through the coelomic fluid while the funicular system provides some of the nutrient transport. The funicular system is a transport system that connects between individual zooids of the colony at specialized regions of the wall called pore plates (Bobin 1977). Funicular cords are muscle fibres which have a peritoneal covering and cross the trunk of the coelom. The funiculus is a funicular cord which connects the body wall to the stomach (Ruppert et al.,2004).
Some zooids contain dark brown spheres called brown bodies. Brown bodies are a method of storing waste and are the remains of a degenerated zooid polypide (lophophore and gut) and can either remain permanently in the coelom or temporarily incorporated into the new polypide from the unaffected tissues of the cystid (zooecium and bodywall) and then excreted (Ruppert et al.,2004).
|
|
|
Nervous System | |
The zooidal nervous system of bryozoans consists of a small ganglion positioned dorsally between the mouth and the anus and a nerve ring around the pharynx (Ruppert et al., 2004; Ryland 2005). Even though the nerve ring is laterally thick, it has been reduced to a fibrous strand. The nerve ring sends out motor fibres and sensory fibres into each tentacle as well as a nerve net around the body wall (Ruppert et al., 2004). While there are no specific sensory organs, Bugula growth is photopositive, therefore, it is hypothesised that sensory cilia with photoreceptive capabilities occur on each of the tentacles (Ruppert et al., 2004). A study on the bryozoan gymnolaemate Membranipora, showed that when stimulated zooids will retract their lophophores and instantaneously cause a nearby response in other zooids; this allows nerve impulses to be time recorded (Ryland1979; Ryland 2005).
|
|
|
|
Evolution and Systematics | |
Approximately 15,000 fossilised bryozoan species have been found, dating from the early Ordovician to late Cambrian (Boardman & Cheetham, 1987). However, molecular phylogenetic analyses reveals that bryozoans would have originated earlier during the Cambrian period. Reasons for lack of fossils from this time is due to the earliest bryozoans not being calcified and, therefore, could not fossilise (Fuchs et al., 2011). The order cheilostomes first appear in the fossil record approximately155 mya during the Upper Jurassic (Ostrovsky et al., 2008) and since had become the most dominant bryozoan.
Bryozoa is recognized as being made up of three major clades; Stenolaemata (Cyclostomata), Gymnolaemata (Eurystomata), and Phylactolaemata but the interrelationship between these clades is unknown (Fuchs et al., 2009; Waeschenbach et al., 2009). The phylogenetic position of Bryozoa is also ambiguous (Helmkampf et al., 2008; Paps et al., 2009). Bryozoans, brachiopods and phoronids are generally classed in Lophophorata because they share the distinct lophophore (Ruppert et al., 2004). However, the Lophophorata classification is one of the most controversial issues in metazoan phylogeny (Jang& Hwang, 2009). This is because Lophophorata have a combination of protostomeand deuterostome characteristics (Jang & Hwang, 2009). Morphological and larval features are the basis of deuterostome classification (Conway-Morris 1995). However, through molecular phylogenetic analysis there is a relationship between lophophorates and several lophophorates including molluscs and annelids (Conway-Morris et al., 1996; Passamaneck & Halanych, 2004). Figure 7 shows one possible placement of bryozoans in a phylogentic tree, as well as showing Bugula neritina with its sister taxa Flustrellidra hispida.
|
 |
| Figure 8 |
|
|
|
Biogeographic Distribution | |
B. neritina is a colonial invertebrate which is common in temperate waters worldwide (Yu et al., 2007). They are found to densely colonise any openly available hard substrate including many artificial submerged structures and surfaces, and vessel hulls (Fehlauer-Ale et al., 2014; Mackie et al., 2006). It is one of the most abundant bryozoans in harbours, ports and embayments, and an important fouling organism (Keough & Ross, 1999). It was first recorded in Victoria, Australia in 1881 (Keough & Ross, 1999) and is now widespread in Australian harbours (Mackie et al., 2006).
|
|
|
Conservation and Threats | |
B. neritina are abundant and occur worldwide predominately in ports and harbours. While their population is unknown, they are not at risk. However, they are a highly invasive species which dominate polluted areas and out compete native species for space and nutrients. Several studies have suggested that invasive species compared to natives can tolerate higher concentrations of metal (i.e. copper) (Crooks 2005; Johnston & Keough, 2002; Hall 1981; Piola & Johnston, 2006). With increasing metal contamination the diversity of native species in an area decreases, while invasive species were generally unaffected. In areas with low pollution, less tolerant native species are able resist invasions. From these results it can be seen that there is a need to improve coastal water quality in regards to heavy metal pollutants especially around harbours and urbanised areas.
|
|
|
References | |
Avila, C (1995). Natural products from opisthobranch mollusc: a biological review. Oceanography and Marine Biology Annual Review, 33: 487-559.
Bishop, J., Pemberton, A (2006). The third way: spermcast mating in sessile marine invertebrates. Integrative and Comparative Biology, 46: 398-406.
Boardman, R., Cheetham, A (1987). Phylum Bryozoa. In: Boardman, R., Cheetham, A., Rowell, A (eds), Fossil Invertebrates. Blackwell Scientific Publications, Boston, p. 497-549.
Bobin, G (1977). Interzooecial Communications and the Funicular System. Woollacott, R., Zimmer,R. (Eds.). Biology of Bryozoans. Academic Press, New York.
Burgess, S., Hart, S.,Marshall, D (2009). Pre-Settlement Behavior in Larval Bryozoans: The Roles of Larval Age and Size. Biological Bulletin, 216: 344-354.
Carlton, J., Geller. J (1993). Ecological roulette: the global transport of nonindigenous marineorganisms. Science, 261: 78-82.
Carte, B., Faulkner, D (1986). Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan. Journal of Chemical Ecology, 12: 798-804.
Conway-Morris, S (1995). Nailing the Lophophorates. Science, 375: 365-366.
Conway-Morris, S., Cohen, B., Gawthrop, A., Cavalier-Smith. T,, Winnepenninckx, B (1996). Lophophoratephylogeny. Science, 272: 282.
Crooks, J (2005). Oral commentary. Vessel Fouling Technical Advisory Group, October 13, 2005: Meeting Summary. California State Lands Commission, Sacramento, CA.
Dahms, H., Dobretsov, S., Qian, P (2004). The effect of bacterial and diatom biofilms on the attachment of the bryozoan Bugula neritina. Journal of Experimental Marine Biology and Ecology, 313:191-209.
Davidson, S., Haygood, M (1999). Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbour distinct strains of the bacterial symbiont "Candidatus Endobugula sertula". Biological Bulletin, 196: 273-280.
Davidson, S., Allen, S., Lim, G., Anderson, C., Haygood, M (2001). Evidence for the biosynthesis of bryostatins by the bacterial symbiont "Candidatus Endobugula sertula" of the bryozoan Bugula neritina. Applied Environmental Microbiology, 67: 4531-4537.
Dyrynda, P., Ryland, J (1982).Reproductive strategies and life histories in the cheilostome marine bryozoans Chartella papyracea and Bugula flabellata. Marine Biology, 71: 241-256.
Fehlauer-Ale, K., Mackie, J., Lim-Fong, G., Ale, E., Pie, M., Waeschenbach, A (2014). Cryptic species inthe cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zoologica Scripta, 43: 193-205.
Fuchs, J., Obst, M., Sundberg, P (2009). The first comprehensive molecular phylogeny of Bryozoa (Ectoprocta) based on combined analysis of nuclear and mitochondrial genes. Molecular Phylogenetics Evolution, 52: 225-233.
Fuchs, J., Martindale, M., Hejnol, A (2011). Gene expression in bryozoan larvae suggest a fundamental importance of pre-patterned blastemic cells in the bryozoan life-cycle. EvoDevo, 2: 13.
Gordon, D (1974). Microarchitecture and function of the lophophore in the bryozoan Cryptosula pallasiana. Marine Biology, 27: 147-163.
Hall, A (1981). Copper accumulation in copper-tolerant and non-tolerant populations of the marine fouling alga, Ectocarpus siliculosus (Dillwyn) Lyngbye. Botanica Marina, 24: 223-228.
Helmkampf, M., Bruchhaus, I., Hausdorf, B (2008). Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proceedings of the Royal Society B: Biological Sciences, 275:1927-1933.
Jackson, J., Wertheimer, S (1985). Patterns of reproduction in five common species of Jamaicanreef-associated bryozoans. In: Nielsen, C., Larwood, G (eds), Bryozoa: Ordovician to recent. Olsen and Olsen, Fredensborg, pp 161-168.
Jang K., Hwang, U (2009).Complete mitochondrial genome of Bugula neritina (Bryozoa, Gymnolaemata, Cheilostomata): phylogenetic position of Bryozoa and phylogeny of lophophorates within the Lophotrochozoa. BMC Genomics, 10: 167.
Jebram, D (1979). Interrelations of nutrition, food uptake, and growth in bryozoans. In: Larwood,G., Abbott, M (eds) Advances inbryozoology Systematics Association Special, Volume No 13, Academic Press, London, p 121-140.
Johnston, E., Keough, M (2002). Direct and indirect effects of repeated pollution events of marinehard-substrate assemblages. Ecological Applications, 12: 1212-1228.
Keough, M (1989). Variationin Growth Rate and Reproduction of the Bryozoan Bugula neritina. Biology Bulletin, 177: 277-286.
Keough, M., Chernoff, H (1987). Dispersal and population variation in the bryozoan. Ecology, 68: 199-210.
Keough, M., Ross, J (1999). Introduced fouling species in Port Phillip Bay. In: Hewitt, C., Campbell, M., Thresher, R., Martin, R (Eds.). Marine Biological Invasions of Port Phillip Bay, Victoria. Centre for Research on Introduced Marine Pests, CSIRO Marine Research. Hobart, Tasmania, pp. 193-225.
Lindquist, N., Hay, M (1996). Palatability and chemical defense of marine invertebrate larvae. Ecological Monographs, 66: 431-450.
Loeb, M., Walker, G (1977). Origin, composition, and function of the secretions from pyriformorgans and internal sacs of four settling cheilo-ctenostome bryozoan larvae. Marine Biology, 42: 37-46.
Mackie, J., Keough, M.,Christidis, L (2006). Invasion patterns inferred from cytochrome oxidase Isequences in three bryozoans, Bugula neritina, Watersipora subtorquata; and Watersipora arcuata. Marine Biology, 149:285-295.
McGovern, T., Hellberg, M (2003). Cryptic species, cryptic endosymbionts, and geographic variation in chemical defenses in the bryozoan Bugula neritina. Molecular Ecology, 12: 1207-1215.
Miller, M (1961). The food of nudibranchs. Journal of Animal Ecology, 30: 95-116.
Ostrovsky, A., Taylor, P., Dick, M., Mawatari, S (2008). Pre-Cenomanian Cheilostome Bryozoa: Current State of Knowledge. Origin and Evolution of Natural Diversity, Proceedings of International Symposium, Sapporo, pp.69-74.
Paps, J., Baguñà, J.,Riutort, M (2009). Bilaterian phylogeny: a broad sampling of 13 nuclear genes provides a new lophotrochozoan phylogeny and supports a paraphyletic basal Acoelomorpha. Molecular Biology and Evolution, 26: 2397-2406.
Passamaneck, Y., Halanych,K (2004). Evidence from Hox genes that bryozoans are lophotrochozoans. Evolution & Development, 6: 275-281.
Pearse, V., Pearse, J., Buchsbaum,M., Buchsbaum, R (1987). Living Invertebrates. Blackwell Science, Cambridge, Massachusetts.
Piola, R., Johnston, E (2006).Differential resistance to extended copper exposure in four introduced bryozoans. Marine Ecology Progress Series, 311: 103-114.
Pratt, M (2004). The effect of zooid spacing on bryozoan feeding success: is competition or facilitation more important? Biological Bulletin, 207:17-27.
Riisgard, H., Manriquez, P (1997). Filter-feeding in fifteen marine ectoprocts (Bryozoa): particle captureand water pumping. Marine Ecology Progress Series, 154: 223-239.
Ruppert, E., Fox, R., Barnes, R (2004). Invertebrate Zoology: A Functional Evolutionary Approach, (7th Ed). Brooks/Cole Thompson Learning,Belmont, California.
Ryland, J (1976). Physiology and ecology of marine bryozoans. Advances in Marine Biology, 14: 285-443.
Ryland, J (1979). Structural and physiological aspects of coloniality in Bryozoa. In: Larwoodg, P., Rosen, B (Eds.): Biology and Systematics of Colonial Organisms. Academic Press, London, New York: 211-242.
Ryland, J (2005). Bryozoa: an introductory overview. In: Woess, E (ed) Moostiere (Bryozoa), Moss Animals (Bryozoa). Denisia vol 16, Biologiezentrum Linz, Landesmuseen Neue Serie 28, pp 9-20.
Ryland, A., Bishop, J., DeBlauwe, H., Nagar, A., Minchin, D., Wood, C., Yunnie, A (2011). Alien species of Bugula (Bryozoa) along the Atlantic coasts of Europe. Aquatic Invasions, 6: 17-31.
Sharp, K., Davidson, S.,Haywood, M (2007). Localization of 'Candidatus Endobugula sertula' and the bryostatins throughout the life cycle of the bryozoan Bugula neritina. ISME Journal, 1: 693-702.
Singh, R., Sharma, M.,Joshi, P., Rawat, D (2008). Clinical status of anti-cancer agents derived frommarine sources. Anticancer Agents in Medicinal Chemistry, 8: 603–617.
Sutherland, J., Karlson, R (1977). Development and stability of the fouling community at Beaufort, NorthCarolina. Ecological Monographs, 47:425-446.
Waeschenbach, A., Cox, C., Littlewood, D., Porter, J., Taylor, P (2009). First molecular estimate of cyclostome bryozoan phylogeny confirms extensive homoplasy among skeletal characters used in traditional taxonomy. Molecular Phylogenetics and Evolution, 52: 241-251.
Walters, L., Miron, G.,Bourget, E (1999). Endoscopic observations of invertebrate larval substratum exploration and settlement. Marine Ecology Progress Series, 182: 95-108.
Winston, J (1977). Distribution and Ecology of Estuarine Ectoprocts: A Critical Review. Chesapeake Science, 18: 34-57.
Woollacott, R., Zimmer, R (1971). Attachment and metamorphosis of the cheilo-ctenostome bryozoan Bugula neritina (Linne). Journal of Morphology, 134: 351-382.
Yu, X., Yan, Y., Gu, J(2007). Attachment of the biofouling bryozoan Bugula neritina larvae affected by inorganic and organic chemicalcues. International Biodeterioration & Biodegradation, 60: 81-89.
Zardus, J., Nedved, B., Huang, Y., Tran, C., Hadfield, M (2008). Microbial Biofilms Facilitate Adhesion in Biofouling Invertebrates. Biology Bulletin, 214: 91-98.
|
|
|
|
|