Life History & Behaviour
Respiration
Respiration and gas exchange is achieved through the use of tube feet and five pairs of peristomial gills. Tube feet in echinoids act as gills, carrying water to and from the water-vascular system and perivisceral cavities (Ruppert et al, 2004). These tube feet contain two canals separated by a septum, which prevents diffusion between the incoming oxygen rich water and the outgoing oxygen deficient water. Muscles from Aristotle’s lantern, the protrusible jaw apparatus, are used to pump coelomic fluid to and from the peristomial gills, and these gills are believed to be the main gas exchange surface for this structure (Ruppert et al, 2004)

Figure 1. Cross section of tube feet, displaying the current flow within the foot (Rupert et al, 2004).
Locomotion
As with other echinoderms, E.mathaei moves using tube feet. These tube feet are thin tentacle-like arms protruding from the ambulacural plates, ending in a sucker. By moving these tube feet forward, gripping the substrate, retracting the foot and then releasing, the urchin can move across the substrate (Ruppert et al, 2004). Movement of the tube feet is performed by contractions within the ampullae, a small muscular sack at the base of the tube foot. Urchins are able to move in any direction, using any ambulacural area to lead. These urchins are also able to raise and lower themselves onto the substrate by moving their spines.

Figure 2. E.mathaei with tube feet extended.
Figure 3. E.mathaei locomotion using tube feet
Reproduction
Echinometra mathaei are gonochoric and reproduce by releasing eggs and sperm into the seawater, where external fertilization occurs. The larvae of these urchins develop in the plankton, and have a characteristic larval form known as echinopluteus(R McEdward and G Miner,2007).Once these larvae reach competency, settlement may be induced if certain environmental cues are found, and the larvae will quickly metamorphose. Studies have found that E.mathaei has a seasonal reproductive cycle, where breeding is at its peak during the summer months (Muthiga and Jaccarini, 2005); however in several populations, numerous mature gametes have been found in E.mathaei throughout the year, suggesting that breeding can occur at any time (Drummond, 1995).
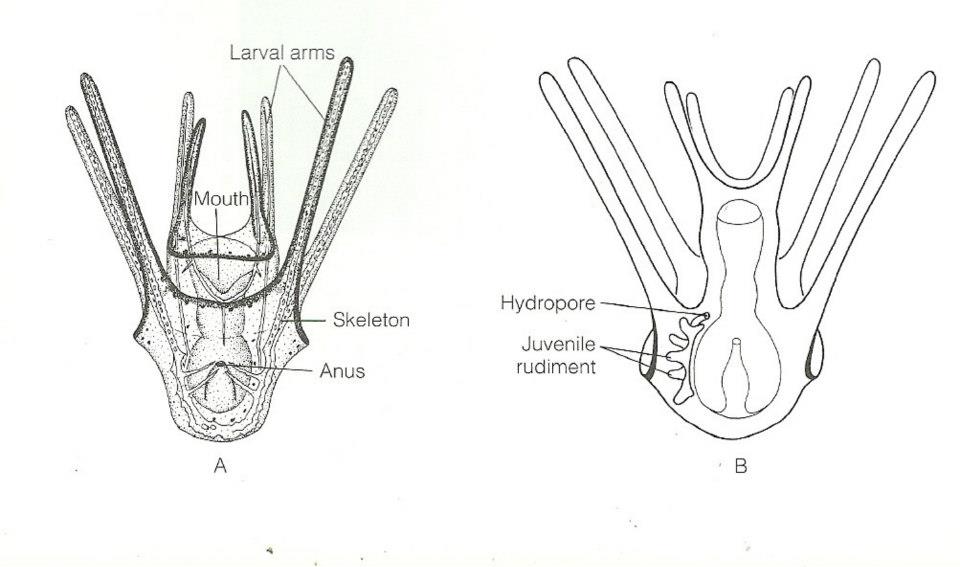
Figure 4. Body plan of echinopluteus larvae (Ruppert et al, 2004).
Feeding
Feeding in most echinoids occurs through the use a protrusible jaw apparatus known as Aristotle’s lantern. This structure consists of five calcareous plates, joined together by retractable muscles, forming a sharp beak composed of 5 teeth. Aristotle’s lantern is supported by strong musculature that allows it to protrude and retract, as well as move from side to side and allow the teeth to open and close (Ruppert et al 2004). This structure allows the urchin to graze on algae growing over hard substrates, and this grazing can lead to erosion of the substrate.
E.mathaei is omnivorous, feeding on a wide range of materials. These urchins have been seen feeding on sea grasses, macroalgae, calcareous algae, sponges, corals and molluscs (Hart and Chia, 1990). The feeding behaviour of these animals is known to vary with some urchins actively foraging for food, while others remain sedentary and rely on snaring drift material (McClanahan and Muthiga, 2007). Most feeding by these urchins is on algae on the surface of hard substrates. Through the use of Aristotle’s lantern, these urchins rasp off algae, and in doing so, they also remove the surface of the coral on which the algae is growing. When these urchins are present in high densities, this feeding can lead to large scale destruction of corals (Downing and El-Zahr, 1987).

Figure 5. Diagram of Aristotle's lantern (Ruppert et al, 2004)
In order to gather more information on the feeding behaviour of these urchins, a study was conducted during the Marine Invertebrates (MARS3211) field trip to Heron Island in September 2012.
Introduction
The tropical sea urchin Echinometra Mathaei, is an omnivorous urchin found throughout the tropical Indo-Pacific region. While this urchin is known to consume various types of food, the most common food source for these urchins is turf algae (Hart and Chia, 1990). The feeding behaviour of these urchins is well studied throughout the world, however there is no data on E.mathaei on Heron Island. As the environmental conditions in the Great barrier reef change, the abundance distribution of different algae species changes, and as algae is a major component of the E.mathaei diet, it is important that we understand as much about their behaviour as possible. This study aims to determine if these urchins show a preference for a particular type of algae found on the reef at Heron Island.
Materials and methods
This study was conducted at the Heron Island research station, from the 18th to the 21st of September, 2012. In this study, 8 specimens of E.mathaei were collected from the reef crest at an area known as A/B reef (23ᵒ43S, 151ᵒ93E), to the north east of the research station. Specimens of four common algae, Hypnea spinella, Padina sp., Hydroclathus clathratus and Colpomenia sinuosa were harvested. The urchins were transported to HIRS where they were placed in a tank with running seawater. Urchins were denied access to food for 24 hours prior to the experiment. Before the commencement of the study, all algae was washed with clean seawater to remove any other organic matter.
Eight 10cm deep trays were set up with approximately 8cm of seawater in each tray. The different algae species were placed in each corner of each tray and algae positions were rotated one position clockwise for each additional tank, to control for any inherent biases in urchin movement. At the beginning of each trial, an urchin was placed in the centre of each tray. Experimentation commenced at 9pm to allow for the natural nocturnal feeding behaviour of these urchins.
Urchin movement was monitored, and when an urchin reached a species of algae in the corner of a tray, this was recorded as the urchin’s preference for that trial. After all urchins had shown a preference, the trays were drained and set up again. Urchins were then placed in the centre of the adjacent tray in a clockwise direction and then next trial was conducted. This process was repeated for a total of 8 trials, with a total of 64 replicates. Analysis of data was done by the use of an ANOVA.
Results
In each trial, urchins took between 5 and 20 minutes to reach a particular species of algae. In all 8 trials, 2 urchins did not show any movement, and trials were ended after 25 minutes of inactivity. These urchins were not included in the data, resulting in a total of 48 replicates. Urchins appeared to show a preference for Padina sp., however this was found to be insignificant (p=0.344).
Discussion
It was expected that if a particular species of algae was preferred or disliked more than the others, this would be reflected in the data. While the data suggested that Padina sp. was the favourite and H.clathratus was least preferred, the results were insignificant. The insignificance of these results may be explained by the fact that the stagnant water in each tray may have allowed for mixing of the chemical scents given off by these algae species, confusing the urchin as to which direction each species lies. Future studies conducted in tanks specifically designed to prevent scent mixing may provide better data. As urchins are known to feed either by grazing on algae on hard substrates, or by snaring drifting algae (McClanahan and Muthiga, 2007), we should also attempt to gain an understanding of which method E.mathaei on Heron Island prefer. If these urchins prefer one feeding method over another, this experiment may have not have been suited for an algae preference study.
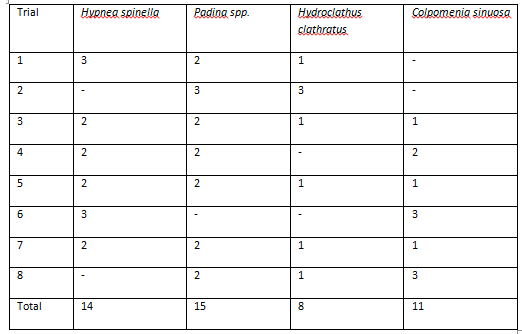
Figure 6. Table of urchin algae preferences. Values represent the number of urchins selective each algae in each trial (p=0.344).

Figure 7. E.mathaei feeding on Padina sp.

Figure 8. Set up of study, displaying the different algae configuration in each tray. |