Life History & Behaviour
The close association between crabs of thegenus T
rapezia and Pocilloporid corals has led to some veryunique and interesting life-history characteristics and behaviour.
Feeding:
Trapezia crabs symbiosis with Pocillopora corals not only provides them with a place to shelter from the predators and the elements, it also provides them with their food source. These crabs feed on the mucus secreted by the corals (Knudsen, 1967, Patton, 1974). They use “food brushes” and “food combs” located on the terminal segments (dactylus) of each walking leg (Knudsen, 1967) (fig. 1 & 2). The food brushes are located on the distal (away from the body) end of each walking leg dactylus and are composed of short, blunt ended spines for agitating the coral polyp and a dense tuft of bristles for collecting the mucus or other debris that might fall onto the coral (Knudsen, 1967) (fig. 1 & 2). The food comb is located on the inside surface of each dactylus and is composed of a number of rows of feathered bristles (setae) (Knudsen, 1967) (fig. 1 & 2). The number of rows varies in each species; T. cymodoce has 6 to 8 rows (Castro, 1997a). These food combs are used to concentrate the coral mucus and pass it to other legs or mouthparts. The three sets of mouthparts (maxillipeds) also contain bristles for collection and movement of mucous. In addition to coral mucous Trapezia crabs also feed on sediment and detritus that fall onto their coral hosts (Patton, 1974).

Figure 1: Close-up of the dactylus of the 1st walking leg of a T. cymodoce specimen
showing the "food brush" and "food comb"

Figure 2: Diagram illustrating Food brush and Food comb or Trapezia crabs
Adapted from Knudsen (1967)
Symbiosis with Pocillopora colony:
The fact that Trapeziid crabs feed on coral mucus suggests they might be an ectoparasite on coral hosts (Knudsen, 1967). However, there has been evidence that shows corals may also benefit from the relationship with the crabs providing a number of services. Firstly, Trapeziid crabs help remove sediment from coral hosts, which promotes survival and growth of corals. It was shown in a field experiment that removal of Trapeziid crabs resulted in slower growth, increased tissue bleaching and sediment load and an increase in mortality of up to 45-80% (Stewart et al., 2006). Secondly, Trapeziid crabs have been shown to defend their host Pocillopora colony from Crown-of-Thorns Starfish (Acanthaster planci) by snipping spines and tube feet of the invading starfish (Glynn, 1976) (see video below). Rather than the crabs being an ectoparasite on the corals it is clear that it is a mutual relationship where one receives food while the other receives a private cleaner and security guard.
Video: Showing a T. cymodoce defending its Pocillopora host
Video shot by youtube user jsstella12
Reproduction:
Adults of the species of Trapezia almost always occur in heterosexual pairs, with a single pair per coral colony (Austin et al., 1980, Patton, 1966, Patton, 1974, Castro, 1997a). The formation of mating pairs and the association with Pocillopora corals means that Trapezia crabs have access to mates, food and shelter all year round. This enables a significant investment into reproduction which is shown by the quick generation time with females generally producing a new clutch of eggs 1-2 days after the previous clutch has hatched (Huber and Coles, 1986, Stella et al., 2011a). Reproduction is year-round and in T. cymodoce clutch sizes can be over1,500 eggs, with each egg about 0.35mm in diameter (Stella et al., 2011a, Huber, 1983) (fig. 3). Additionally, egg clutch size increased considerably with increase in female carapace width, providing an adaptive advantage of larger size in females (Huber, 1983, Stella et al., 2011a). Once eggs hatch the larvae enter the plankton where they disperse and remain for a length of time before settling onto a coral host. Little is known about the dispersal stage of Trapezia.
   
Figure 3: Images of the egg clutch of a female T. cymodoce stored on the ventral side of the abdomen
Movement:
Long range dispersal of T. cymodoce occurs during their planktonic larvae stage which provides a mechanism to explain how such a small critter has such a wide distribution. In addition to this larval dispersal stage, settled T. cymodoce individuals also show considerable movement between Pocillopora colonies (Stella et al., 2011a, Huber, 1983, Patton, 1974). Movement typically occurs at night when risk of predation is reduced and involves individuals moving to more desired Pocillopora colonies; larger and more healthy (i.e. not damaged or bleached) (Stella et al., 2011a). Mate selection may also govern movement in males between coral heads, with males typically preferring larger more fecund females (Huber, 1983).
Heron Island Project:
Introduction
Crabs of the genus Trapezia are small obligate symbionts of Pocillopora spp. Coral (Patton, 1966, Patton, 1974, Castro, 1997). Adults crabs are almost always foundin heterosexual pairs living within the branches of a Pocillopora host colony (Patton, 1974). Studies have been done on a range ofspecies of Trapezia looking at thedensity and abundance of these species in coral heads of differing size (Patton, 1974, Huber, 1983). It is also known that these crabsmigrate between coral heads and pairs are territorial of their inhabited coralhead, warding of invading crown-of-thorns starfish as well as would be competitorsof the same species trying to usurp their position (Stella et al., 2011, Glynn, 1976). However, it is only assumed thatcrabs migrate in order to inhabit larger coral heads, so far no study has beendone to test the controlling factors of these migrations.
The aim of this study was to determine if therewas a preference by Trapezia crabs ofthe species Trapezia cymodoce for Pocillopora coral colonies based oncolony size and quality.
Three experiments were conducted to test this.In experiment 1, we hypothesized that T.cymodoce would prefer coral heads of large and good quality compared tosmall and bad quality coral heads. Experiment 2, we hypothesized that male T. cymodoce crabs would migrate fromsmall coral heads to large coral heads. Experiment 3, we hypothesized that theintroduction of female T. cymodocewould lead to increased occurrence of male migration from small to large coralheads and between coral heads of the same size.
Method
Study site:
The studysite was at Heron Island Reef in the southern Great Barrier Reef. Theexperiments were conducted within the laboratory facilities within theUniversity of Queensland Heron Island Research station. The study was conductedover a five day period from September 16th to 21st.
Collection of crabs:
T. cymodoce and their host Pocillopora coral head specimens werecollected from the reef flat adjacent to Heron Island reef, in Shark Bay. Coralspecimens with symbiont crabs were removed from the substrate and transportedback to the lab in buckets filled with seawater. Specimens were kept withintanks at the research station with a constant supply of fresh seawater.
Experiment 1:
Involved exposing the crabs to four choices of Pocillopora coral heads and observing which coral head they chose. Four coral heads were chosen: i) Large good quality, ii) Large Bad quality, iii) Small good quality,iv) Small bad quality. Coral heads of the same size class were of very similar size, and quality was determined by the amount of damaged or dying corals in each colony. The four colony heads were place randomly in each corner of the experiment tank, they were reallocated at random following the end of each round. T. cymodoce crabs were placed in the middle of the experiment tank and the coral head they moved to was observed and recorded. Overall 12 crabs were used from 6 pairs (6 male and 6 female) and each were tested three times so n=36.
Experiment 2:
In this experiment T. cymodoce males were placed in small tanks containing two coral heads of similar quality but varying size and left for a period of 4 hours to determine if they would migrate. Six tanks were setup with three treatments and three controls. Treatment tanks contained coral heads of different sizes; one small and one large .Male crabs were placed on the smaller coral head and then left for the period of the experiment. Control tanks contained two coral heads of similar size,male crabs were randomly placed on one of the two coral heads. Following the four hour period coral heads were removed so that it could be determined if the male crab had migrated.
Experiment 3:
This experiment was identical to experiment 2 except that female crabs were added to the opposite corals that the males were placed on. For two of the treatment tanks males were placed on the small coral head and the female was placed on the large coral head. In the third treatment tank, since male migration was observed in experiment 2, I placed the male on the large coral head and the female on the small coral head. In the control treatment, again the male and female crabs were randomly allocated. The tanks were left overnight and then the coral heads were removed in the morning so migration could be determined.
Results
Experiment 1:
Significant differences were found in crabcoral preference in terms of the size of the corals, with larger corals chosenmore often than small corals (p = <0.01) (figure 3). No significantdifference was found for coral preference based only on coral quality (p =>0.05) (figure 4). A significant difference was found in preference based onsize and quality, with Large good quality corals chosen most often (p =<0.01) (figure 5).

Figure3:Preference of T. cymodoce based on Pocillophora colony size

Figure4:Preference of T. cymodoce based on Pocillophora colony quality
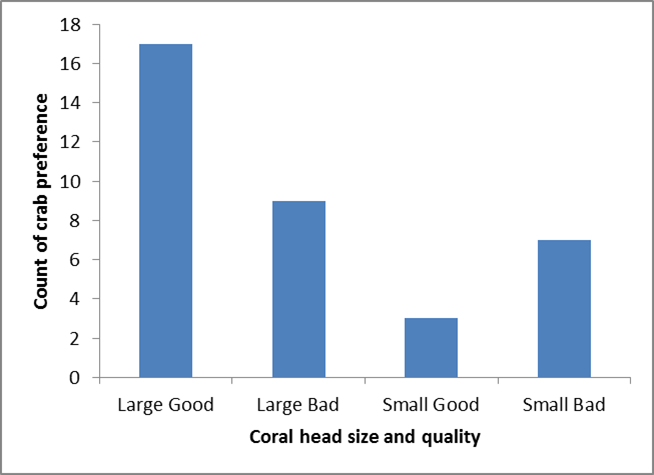
Figure5:Preference of T. cymodoce based on Pocillophora colony size and quality
Experiment2:
Only two males migrated during the 4 hourstudy period; one from the control and one from the treatment group.
Experiment3:
On all the treatments migration occurred fromthe small coral head to the large coral head. This involved two male migrationsand one female migration. Additionally, one male migrated within the controltanks.
|