|
|
|
Holothuria scabra: Exhibiting Beauty Through Slow-moving Grace
|
|
Madison Loos 2015
|
|
|
|
Summary | |
Holothuria scabra is a common sea cucumber within the Holothurian class. They were first discovered by Jaeger in 1833. These cucumbers are most commonly called sandfish world wide due to their sand burrowing lifestyle but have a large variety of names depending on the region they are found. They are characterized by being grey to black or olive in color with dark transverse lines across their dorsal side. H. scabra are exploited for trade on the bêche-de-mer market as they are sold as an Asian delicacy. Conservation of these animals is of high concern as their wild populations are plummeting.
|
|
|
Physical Description |
General Description | |
H. scabra are a part of the Holothurian class within the phyla Echinodermata. Holothurians we given the name sea cucumbers as they have a cylindrical body shape and are tapered at the end. They are more often than not slimy and have a pliable body. They share with their sister classes the
pentaradial body symmetry and a water vascular system. Unlike the other classes of Echinoderms, Holothurians express their pentaradial symmetry horizontally rather than
vertically due to the lengthening of the body across the oral-aboral axis (Cannon & Silver, 1987).
The lifestyle and ecology of these
animals drove the evolution of these animals' body plans. H. scabra are
deposit feeders and have elongated bodies with a blunt end. Their mouth parts
are directed downwards towards the surface of the substrate while their anus is
located more dorsally giving it a convex appearance.
|
|
|
Detailed Physical Species Description | |
H. scabra are grey or black to olive in coloration often with black or green transverse lines across the dorsal portion of their body (Figure 1). H. scabra are darker dorsally than they are ventrally which tends to be grey or white in colour and is characterized by having many "speckles" out of which the tube feet extend (Figure 2). These ventral tube feet are used for movement.

Figure 1. Dorsal view of Holothuria scabra. Transverse lines apparent as well as grey to olive coloration. 
Figure 2. Ventral side of Holothuria scabra pressed up against glass; animal oriented with oral mouth parts to the furthest right of the photo. Light gray coloration contrasts darker olive dorsal side. Speckles that house tube feet are apparent.
 Figure 3. Up close view Holothuria scabra exhibiting the dorsal papillae and the ventral tube feet. The animal's mouth is oriented to the right most portion of the photo.
Figure 3. Up close view Holothuria scabra exhibiting the dorsal papillae and the ventral tube feet. The animal's mouth is oriented to the right most portion of the photo.
The coloration of H. scabra makes them quite conspicuous within coral rubble or debris or within sand in sea grass beds (Figure 4).
 Figure 4. Holothuria scabra against coral rubble to exhibit similar and cryptic coloration.
Figure 4. Holothuria scabra against coral rubble to exhibit similar and cryptic coloration.
As adults H. scabra measure between 150 and 400 mm and may weigh up to between 500 and 2000 g but this varies dramatically between geographical ranges (Conand, 1989). Their distribution within a region varies between life stages (see settlement and
distribution)
.
|
 |
| Figure 1 |
|
 |
| Figure 2 |
|
 |
| Figure 3 |
|
 |
| Figure 4 |
|
|
|
|
Ecology |
Settlement and Distribution | |
The most important aspect of where H. scabra is found, is the place of settlement of the species’
larvae. There is strong evolutionary pressure to settle in a location that may
increase chance of growth and survival (Giese et al., 1991). Hypothesises have been stated that H. scabra larvae are highly selective
when it comes to settling on a substrate. Mercier et al. (2000) found that H.
scabra selectively settle upon sea grasses found within the adult habitat
and larval settlement should be correlated with levels of sea grass within a
habitat. As well, their data showed that larval settle more upon sands that are
higher in organic material and contain coral rubble. In conclusion, sea grass
beds are important to H. scabra settlement
and early life activity.
H. scabra individuals
are found in different locations within a region correlated to its size. Research
done by Mercier (2000) showed that distribution of individuals is
correlated with habitat characteristics such as depth, granulometry, richness
of substrate, and presence of sea grass beds. This studies results indicated
that larger individuals are found in deeper waters and in less dense sea grass
beds due to the increased food availability in these areas; dense sea grass
beds have root systems that could interfere with the larger individuals burrowing
cycle (Mercier 2000). As well, deeper water tends to be more stable in
temperature and other variable factors and larger individuals tend to be more
sensitive to change than smaller individuals. In addition to these factors, H. scabra compromise their depth in
relation to their preferred substrate. These animals prefer fine sediment without
crushed shells and under 30% organic material within the sediment (Mercier
2000). There is a compromise that has to be made as finer sediment may be
found in deeper waters but more organic material may be found in the shallower
areas. The results of this study helps explore the reasons as to why different
sizes of H. scabra may be found at
different depths.
|
|
|
Ecological Role | |
H. scabra are
often closely associated with sea grass beds as they need them for larval
settlement, early life stages, and food production. It is through this
connection, many studies have assessed the importance of the presence of H. scabra in relation to sea grass bed
and ecosystem maintenance. They play a large role in the nutrient availability within
an ecosystem.
Through their burrowing cycle and deposit feeding H. scabra are considered important contributors
to bioturbation or the moving and disturbance of sand and other particles (Wolkenhauer,
2008). These deposit feeders may have a direct impact on the productivity of an
area due to nutrient recycling within the sediment and water column (Moriarty et al., 1985). Hughes et al. (2004) found that nutrient availability
is a limiting factor to growth and success of sea grass beds. Wolkenhauer
(2004) looked to determine the relationship between sea grass productivity and H. scabra
presence. Sea grass productivity was found to be lower without the sea cucumbers
present. The researchers hypothesized that there was stress to the sea grass
through increased shading as organic material built up and decreased nutrient
availability. This relationship between sea grass productivity and sea cucumber
presence is thought to be tied to the bioturbation that sea cucumbers perform. The
bioturbation causes nutrients to become more available and accessible to
seagrasses (Wolkenhauer, 2004). Through these results it can be inferred that
sea cucumbers play an important ecological role within their environment.
|
|
|
|
Life History and Behaviour |
Reproduction and Life Cycle | |
H. scabra have separate
sexes that with no visual sexual dimorphism. It is almost impossible to
identify the sex without internal or microscopic investigation (baskar, 1994). In
both sexes the gonads are yellow, branching, and hang freely. They have a
gonoducts that opens externally near the anterior end that is used for spawning.
In females the gonadial tubules are short and wide while in males they are
longer (Baskar, 1994). At sexual maturity males will be shorter than females
(Shelley, 1981).
Spawning events happen periodically. However some research
has shown that spawning may occur all year around (e.g., Krishnan, 1968). However,
there is also extensive research that shows there is at least two major
spawning peaks within the year (MPEDA, 1989). During spawning, H. scabra will lift the anterior end of
its body and make a sweeping motion. During this time, the gonad papilla will
deflate (Conand, 1993). The gametes will be released through the gonoporeand into the water column (Mary Bai, 1980). Research suggests that
temperature and salinity may be the triggers for gamete release (Moiyadeen, 1994).
Variation in these factors may trigger the spawning events depending on their
timing within the year.
Once the adult H.
scabra spawn, the eggs and sperm meet. From there division occurs where
within three days a feeding larvae or auricularia is formed. After up to eight days
of feeding, the auricularia undergoes metamorphosis into a non-feeding
dollolaria. At this point, the non-feeding larvae spends up to ten days finding
the best location to settle. At settlement the dollolaria undergoes metamorphosis
into the benthic form of the pentactula. From here tube feet and tentacles
become more apparent and the tables in the body wall begin to form (D. B. James
et aL, 1988). From settlement the juvenile sea cucumber begins to grow and
migrates further from the sea grass bed in correlation to its growth rate (See
Settlement and Distribution).
|
|
|
Feeding Behavior | |
H. scabra are deposit feeders consuming
sediment and organic material from the substrate. Their mouth parts are faced
downwards and they feed while moving slowly over the substrate or sometimes
while they are buried. They use their mucus covered peltate tentacles to extend
and stick to sand particles (Figure 5).

Figure 5. View of H. scabra peltate feeding tentacles within mouth cavity
Once the tentacle has food particle, the animal retracts their tentacle
back into their mouth and removes the particles from their tentacles (Baskar,
1994). Through analysis of their gut contents, it was observed that H. scabra consume mud, sand, shell
debris, molluscan shells, and some biotic material. Through this analysis, it
was also determined that H. scabra
prefers to feed on muddy substrate over sandy substrate (Baskar, 1994).
|
|
Timelapse (30 frames per second) of H. scabra feeding in captivity
See: https://youtu.be/TQdUuO-Tx60 |
When limited to
compact substrate or substrate void of sandy materials, H. scabra have been observed feeding on algae and bacteria
materials from hard substrates and tanks (P. S. B. R. James, 1996; Battaglene et al., 1999). This behaviour was also
observed in the aquarium at University of Queensland. A single individual was
placed in a small tank without sandy substrate, the tank however, was full of bothy
leafy and crust algae. The animal had free roam within the tank for 24 hours.
Upon observation, a large amount of algae was seen to be removed from the tank
and four distinct faecal piles were counted as well (Figure 6). This observation
is congruent with previous research that shows H. scabra will consume algae and bacterial materials outside of the
normal sandy substrate.
Figure 6. View of tank after algae consumption.
|
 |
| Figure 5 |
|
 |
| Figure 6 |
|
|
|
Feeding and Burrowing | |
Feeding rates and times have been
highly discussed among researchers. Some researchers believe that H. scabra are continuous feeders (e.g.,
P. S. B. R. James, 1996) while other research shows that the animals only eat
while they are completely un-burrowed and burrowing halts the feeding process
(e.g., Mercier et al., 1999). Some
research showed that since absorption of organic material takes place in the
stomach and when dissected most animals have empty esophagus and stomachs but full intestines, there must be a stoppage point in these
animals (Baskar, 1994).
Most congruence comes from the notion that
feeding is correlated to burrowing cycles and feeding occurs while exposed on
the surface of the substrate and when movement is occurring. Mercier et al.
(1999) showed that juveniles will spend up to half of their day buried within
the sand and the other half on the surface. Burrowing cycles were disrupted in
individuals that experienced completely 24h darkness. It was hypothesized due
to this research that this burrowing regimen was due to higher predation risk
of the juveniles during the day and they therefore spend more time buried
during daytime hours. It was also hypothesized that burrowing cycles and therefore feeding cycles are affected by
light changes.
Night time feeding was observed
within the aquarium at University of Queensland. A single individual was placed
in a small tank with roughly 25 % of the tank in the darkness while the other
75% was lit by artificial light. The animal had free roam within the tank for
24 hours. The lights of the aquarium are turned off for roughly half of a day. Observations
showed that the animal fed on up to 100% of the substrate in the dark portion
of the tank as characterized by the absence of any algae on the tank surface
post trial (figure 7). As well, it can been seen that although there was
movement within the lit portion of the tank as seen by the fecal piles in the
tank, it does not seem that any significant portion of the algae within the lit
portion was removed (Figure 8). This shows that the animal spent most of its time feeding
within the dark area rather than the lit area. These findings are in line with
the hypothesis proposed in previous research that feeding must be in part influenced
by light change. A hypothetical ‘before’ picture shows what the tank may have
looked like prior to the trail (Figure 9).
Figure 7, 8, 9 From left to right
More research
done by Wolkenhauer (2008) showed that adults also experience diel burrowing
cycles but is not only contingent on light but also on temperature. It was
shown in this study that animals spend more time burrowed as temperatures
decrease and therefore are less active. However, these animals did not change
their affinity to daytime burrowing but instead that they remained burrowed
longer. In addition to this research it has been shown that burrowing cycles
may be affected by stress, tide and current changes, predation, and changes in
salinity and desiccation (Dance et al., 2003;
Mercier et al., 2000; Purcell et al., 2006; Skewes et al., 2000).
|
 |
| Figure 7 |
|
 |
| Figure 8 |
|
 |
| Figure 9 |
|
|
|
Defense | |
H. scabra is a seemingly defenseless animal that is easy for the taking by predators. however, it has multiple defense mechanisms that keep sit safe from consumption. H. scabra house toxins within their body wall tissues that will inflict distress and death in fish and other predators (Rao et al., 1985). Other holothurians have Cuvierian tubules that when excreted attach and hinder predators and therefore save the animal. however, H. scabra has been noted to not posses these tubules. However, some studies have noted that auto-evisceration or the ejection of internal organs, although rare in nature, may occur when the animal experiences either physical or chemical stress (Conand, 1989).
|
|
|
|
Anatomy and Physiology | |
Holothurians we given the name sea cucumbers as they have a
cylindrical body shape and are tapered at the end. They are more often than not
slimy and have a pliable body. Holothurians are like other echinoderm classes as
they have pentaradial symmetry and a water vascular system. Unlike the other
classes of echinoderms, holothurians express their pentaradial symmetry
horizontally rather than vertically due to the lengthening of the body across
the oral-aboral axis (Cannon & Silver, 1986). Within this symmetry, they have five major tracks or
ambulacra that the body’s tissues are associated with. The nervous system,
water vascular system, and haemel system are all organized around these five
tracks. Some families of holothurians also express this pentaradial symmetry
with rows of tube feet mirroring the five major tracks externally (Cannon &
silver, 1986).
Skeleton and ossicles
The skeleton of H. scabra is made up of ossicles that are
characteristic of the Echinodermata phyla. Unlike the other Echinodermata
families, holothurians have ossicles that are reduced to spicules and found dispersed
throughout the body. These ossicles have many forms and shapes specific to
species and therefore species identification is most easily performed through
microscopic analysis of the animal’s spicules. The description of these ossicles can be found in the systematics section.
Calcareous ring
One of the major components of the internal anatomy of the Holothurians is the calcareous ring. This ring is made up of a series of
calcified plates that protects the five major tracks of vital tissues within the
animal. H. scabra’s calcareous ring
is comprised of ten plates that have a large quadrangular radial piece and
sharp anterior v-shaped “teeth” (Massin, 1999). It is through the study of this component of the internal anatomy that species identification and phylogentic relationships can be inferred.
Cuvierian tubules
A unique trait of the family Holothuriidea is the presence
of Cuvierian tubules that are found
attached to the respiratory tree and can be expelled through the cloaca when
disturbed. Despite one study by INDAP ET AL 1996 showing that H scabra possess these tubules, many
studies (i.e. Massin, 1999) refute this argument and suggest that H. scabra actually lack this trait. It
is proposed that Indap et al. (1996)
actually misidentified the structure and was explaining an entirely different
holothurian species (Hamel, 2001).
Digestion
The digestive system starts with the mouth and includes an oesophagus, a stomach, descending and ascending small intestines, a large intestine, a cloaca, and an anus (Mary Bai, 1980). The intestine length is only the length of the body in small juveniles but up to three times the body length in medium
or large juveniles (Mercier, 1999). The gut lies within a coelomic cavity (Cannon & Silver, 1986). As the animal inserts food into its mouth, the total time for the food to pass through the gut of H. scabra takes between 30 and 60 min (Mercier et al., 1999a). Once digested, feces are ejected out of the anus in a string of balloon shaped pellets (Yamanouchi, 1939).
|
|
| Timelapse (30 frames per second) of fecal excretion of Holothuria scabra
|
Movement and water vascular system
The body of H. scabra is characterized by having
longitudinal and circular muscles that are very powerful. Generally sea
cucumbers use their tube feet for movement as well as the rhythmic contraction
and release of their muscles via the water vascular system in a worm like fashion. The water vascular
system is shared with the sister classes of echinoderms. However, it is filled
with coelomic fluid instead of sea water as it is closed off from the water
column. The madreporite is located internally rather than externally like in
other Echinoderms. The radial canals are
reduced to five long canals that run longitudinally along the length of the
body through notches in the calcareous ring (Mooi and David 1997). This system runs through the
tube feet and tentacles and control them through movement of the coelomic fluid.
H. scabra move leading with their
oral end across the substrate surface (Cannon & Silver, 1986).
|
|
|
Timelapse video (30 frames per second) of H. scabra movement using rhythmic contraction of muscles and tube feet
|
|
|
|
Evolution and Systematics |
Systematics | |
Systematics are important and used to classify and describe an animal in relation to the other species.
Holothuria scabra
Phyla: Echinodermata
Class: Holothuroidea
Order: Aspisochirotida
Animals in this family are large animals and often cryptic. They have sausage shaped bodies with thick body walls. They are deposit feeders, ingesting sand and organic material within the substrate. These animals have tube feet present outside of modified feeding tentacles (Cannon& silver 1986).
Family: Holothuriidae
These animals range in size from small to large (<100mm to >300mm). their body is generally circular. They are often concealed within rocks or rubble and sometimes half buried in the sand. Most species are most active at night while some animals experience diel activity. There is a large range in colours and external morphology allowing the identification between live animals, however more detailed assessmentshould be done through analysis of their internal anatomy. Animals in this family have singular gonads, separating them from the paired gonad family, Stichopodidea (Cannon & silver 1986; Rowe, 1969).
Genus Holothuria Linnaeus, 1767
These animals are characterized by having tables present amongst the spicules (however very rarely these tables are absent) as well they have a robust, instead of sinuous, calcareous ring. Usually their body is sausage shaped with variation in body wall thickness. Various life styles, preferred depth, and sediment partiality are exhibited within the genus (Cannon& silver 1986).
Subgenera Holothuria (Metriatyla) Rowe, 1969
The subdivision of the Holothuria genus has been widely argued (James, 1995; Pearson, 1913-1914) through the discussion of plates, buttons, rods, rosettes (primitive or not), the importance of anal teeth etc. but the subdivision proposed by Rowe is most widely accepted throughout the field.
The size of these animals ranges from less than 100mm to 200mm. Twenty tentacles surround the mouth which is downward facing towards the surface of the substrate. The body is generally arched in shape with a flat ventral surface. The tables are robust without cruciform holes and the buttons simple and knobbed within the spicules (Cannon& silver 1986; Rowe, 1969).
Species Holothuria(Metriatyla) scabra Jaeger, 1833
Spicule tables are well developed with spires and knobbed buttons within this species. Buttons are highly numerous and often very large. These animals are found within reefs and coastal habitats; generally exposed completely or partially buried (Cannon& silver 1986; Massin, 1999; Rowe, 1969).
|
|
|
Evolutionary Hypothesis | |
By understanding the evolutionary history of an animal, you can understand where its body plan was derived from and its connecting to its sister species. A few hypothesis exist with the dating of Holothurians within fossil records. Some research proposed a phylogeny of Holothuroids through the use of the morphology of the ossicles and calcareous ring elements left behind after decomposition (Kerr and Kim, 2001). This research proposes that Holothurians arose within the Devonian and Aspidochirotida arose within the Triassic with the class Holothuriidae showing no presence until the Jurassic.
However, more recent research looked at the use of micro- and macropalaeontological methods to identify phylogenetic relationships within the Holothurian family (Reich, 2010). It is believed that the first unequivocal Holothurians are dated to the early Darriwilian but the exact time location of Aspidochirotida is hard to identify as the ossicles are not completely representative and there has been no interpretation of the calcareous ring by palaeontologists (Reich, 2000; Reich, 2010). However, it is estimated that Aspidochirotida arose sometime within the upper Ordovician. There is still much debate over the exact date arrival of the ancestral animal of Holothuria scabra but the relationship of the Holothuroid orders is widely agreed upon (Figure 5).
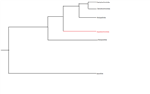
Figure 5. The commonly agreed upon phylogeny of the orders of Holothuroidea. See Kerr (2000) and Reich (2010) for argued time history of these orders.
|
 |
| Figure 10 |
|
|
|
|
Biogeographic Distribution | |
H. scabra is known
in the wild throughout the Indo-Pacific between the latitudes 30ºN and 30ºs
(Massin, 1999) (Figure 11).
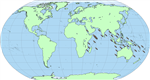
Figure 1 Biographical distribution of Holothuria scabra
Genetic variation is high between
populations of H. scabra within the Indo-Pacific region as distance between the populations acts as a barrier to gene flow (Uthicke
& Purcell, 2004). The species is manly exploited through the Indo-Pacific
as “bêche-de-mer” within Asian markets. Through this exploitation, many
population counts are plummeting within these areas. Mostly large individuals
are sold throughout the Asian markets but where fishing is recorded,
populations are almost completely depleted. Areas where waters are protected
have the highest populations but illegal fishing is often experienced in these
regions causing a population decline (Hasan, 2005).
Holothurians are abundant but most diverse in shallow-water
coral reefs within the tropics however sometimes within temperate waters (Rowe,
1969). H. scabra are characterized
as slow moving animals with low dispersal rates and are often found within low
energy environments especially sea grass beds (Mercier et al., 2000a). Some studies have shown these animals tolerating a
range of salinity even low enough to survive within estuary ecosystems (Mercier
et al., 1999). These animals are mobile and may been seen climbing upon rubble and
throughout the coral debris. H. scabra
are commonly found either fully buried or partially buried in the substrate within sandy regions.
Their distribution within a region is characterized by their size in relation
to depth and organic material within the substrate (see settlement and
distribution).
|
 |
| Figure 11 |
|
|
|
Conservation and Threats | |
Researchers acknowledge the importance to conserve this
species as they play many ecological roles. Not only are they key to
biotubation, they also provide shelter to numerous other animals such as
crustaceans on their external surface or within their body cavity (e.g., Lanchester,
1990). As well, they are some of the key contributors to nutrient recycling
within their ecosystem and therefore play an indirect but pinnacle role in
flora growth (Hughes et al., 2004).
Although the adults are toxic, the auricularia larvae are important prey among
copepods (James et al., 1994)
H. scabra is
becoming of a concern to conservation biologists due to the over exploitation
in many regions worldwide. Populations of these animals are declining rapidly
and is raising concern for their protection (Conand 1990). However implementation of fishing
and protection regulations may be too expensive for developing countries (Conand
and Sloan, 1989). As well, universal protection is too hard to implement as H. scabra are one of the few species of
holothurians that can be sold as first-class bêche-de-mer (Mercier & Hamel,
1997). Due to this, many researchers have studied how to reintroduce cultured
populations into the wild as natural recovery may take too long to conserve the
species (Uthicke & Purcell, 2004).
Restocking the wild may be one of the only ways to maintain
both wild populations and the bêche-de-mer market (Battaglene, 1998). Restocking
can be done by releasing juvenile animals that were cultured in hatcheries. Although
this is a quality method for conservation, it is also expensive. Many countries
are trying to implement this plan through affordable means. As well, ways of
maximizing survival and growth of these animals once released is necessary to
maximize efficiency of these hatcheries (Battaglene and Bell, 1999). As well,
disease is known to wipe out hatchery stocks within weeks of infection (Morgan,
2000). Development of an understanding of bacterial strains that pose a threat
to the species is imperative to maximizing hatchery efficiency
|
|
|
References | |
Baskar B.
(1994) Some Observations on the Biology of the Holothurian Holothuria (metriatyla)
scabra (Jaeger) Central Marine
Fisheries Research Institute, 46, 39-43
Battaglene,
S. (1998). "Can Hatcheries Produce Juvenile Tropical Sea Cucumbers for
Restoration and Enhancement of Wild Stock?"
Battaglene,
S. and Bell, J. (1999). Potential of the tropical Indo-Pacific sea cucumber, Holothuria scabra, for stock
enhancement. In "Stock Enhancement and Sea Ranching" 478--490.
Cannon, L. and Silver, H. 1986. Sea Cucumbers of Northern Australia.
Queensland Museum, South Brisbane, Australia, 60 pp 19-60
Conand, C.
(1989). "Les Holothuries Aspidochirotes du Lagoon de NouvelleCalrdonie:
Biologie, Ecologie et Exploitation". Etudes et Theses, ORSTOM
Conand, C.
and Sloan, N. (1989). World fisheries for echinoderms. In "Marine Invertebrate
Fisheries: Their Assessment and Management" (J. F. Caddy, ed.), 647-663.
Dance S.,
Lane I. and Bell J. (2003). Variation in short-term survival of cultured
sandfish (Holothuria scabra) released
in mangrove-seagrass and coral reef flat habitats in Solomon Islands.
Aquaculture 220(1–4):495–505.
Giese, A., Pearse, J., & Pearse, V.(1974). Reproduction of
marine invertebrates: Echinoderms and Lophophorates., 6
Hasan,
M. (2005). Destruction of a Holothuria
scabra population by overfishing at Abu Rhamada Island in the Red Sea. Marine Environmental
Research,60, 4, 489-511.
James, D.
(1988). Research, conservation and management of edible holothurians and their
impact on the beche-de-mer industry. Central Marine Fisheries Research
Institute, Special Publication 40, 97-98.
James, D. (1995) Taxonomic studies
of the species of holothuria (Linnaeus, 1767) from the seas around India Part I.Journal of The Bombay Natural History Society , 92. 43-62.
James, D.,
Gandhi, A., Palaniswamy, N. and Rodrigo, J.
(1994). "Hatchery Techniques and Culture of the Sea-cucumber Holothuria scabra". Central Marine
Fisheries Research Institute, Special Publication 57, Cochin, India.
Kerr, A. &
Kim, J. (2001). Phylogeny of Holothuroidea (Echinodermata) inferred from
morphology. Zoological Journal of the Linnean Society 133: 63–81.
Krishnan, S. (1968). Histochemical studies on
reproductive and nutritional cycles of the holothuria, Holothuria scabra. Marine Biology 2, 54-65.
Lanchester,
W. (1900). On a collection of crustaceans made at Singapore and Malacca. Part
1. Crustacea Brachyura. Proceedings of the Zoological Society, 719-770.
Mary Bai, M.
(1980). Monograph on Holothuria
(Metriatyla) scabra Jaeger. Memoirs of the Zoological Survey of India 16,
1-75.
Massin,
C., & Nationaal Natuurhistorisch Museum (Netherlands). (1999). Reef-dwelling
Holothuroidea (Echinodermata) of the Spermonde Archipelago (South-West
Sulawesi, Indonesia). Leiden: Nationaal Natuurhistorisch Museum.
Mercier A.,
Battaglene S., and Hamel J.-F. (2000)
Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. Journal of
Experimental Marine Biology and Ecology 249, 89–110.
Mercier, A.
and Hamel, J. (1997). Finding solutions
for beche-de-mer industry. Island Business Magazine September, 44-47.
Mercier, A.,
Battaglene, S., and Hamel, J. (1999). Daily burrowing cycle and feeding
activity of juvenile sea cucumbers Holothuria
scabra in response to environmental factors. Journal of Experimental Marine
Biology and Ecology 239, 125-156.
Moiyadeen, N.
(1994). The biannual reproductive activity in Holothuria (Metriatyla) scabra (Jaeger, 1833), the most abundant
commercial holothuroid of the north-western coastal waters. In " Proceedings
of the First Annual Scientific Sessions NARA", 123-129.
Mooi, R. and David B. (1997). Skeletal homologies of echinoderms.
Paleontological Society Papers 3: 305-355
Morgan, A.
(2000). Aspects of sea cucumber broodstock management (Echinodermata:
Holothuroidea). Secretariat of the Pacific Community Bechede-mer Information
Bulletin 13, 2-8
MPEDA -
Marine Products Export Development Authority (1989). "Sea Weed, Sea Urchin
and Sea Cucumber: Handbook on Aquafarming". Marine Products Export
Development Authority, Kochi, India, pp. 33--47.
Pearson, J.
(1914): Notes on the Holothuroidea of The Indian Ocean. Spo/io zeyhlll. 9 (35):
173- 190.
Rao, D.,
James, D., Girijavallabhan, K., Muthuswamy, S. and Najmuddin, M. (1985).
Biotoxicity in echinoderms. Journal of the Marine Biological Association India
27, 88-96.
Reich, M.
(2010) The early evolution and diversification of holothurians (Echinozoa). Geoscience
Centre of the Georg August University
Reich, M.
2000. The oldest unequivocal record of fossil Holothuroidea. In Anonymous (ed),
10th International Echinoderm Conference, 143.
Shelley, C.
(1981). "Aspects of the Distribution, Reproduction, Growth and Fishery
Potential of Holothurians (beche-de-mer) in the Papuan Coastal Lagoon".
MSc Thesis, University of Papua New Guinea, Papua New Guinea.
Skewes T.,
Dennis D. and Burridge C. (2000). Survey of Holothuria
scabra (sandfish) on Warrior Reef, Torres Strait, in January 2000. CSIRO
Marine Research Final Report
Uthicke,
S., & Purcell, S. (2004). Preservation of genetic diversity in restocking
of the sea cucumber Holothuria scabra investigated by allozyme electrophoresis.Canadian Journal of Fisheries and Aquatic Sciences, 61 519-528.
Purcell S., Blockmans B. & Agudo
N. (2006). Transportation methods for restocking of juvenile sea cucumber,
Holothuria scabra. Aquaculture 251:238–244.
Wolkenhauer S. (2008) The
ecological role of Holothuria scabra (Echinodermata: Holothuroidea) within
subtropical seagrass beds. Journal
of the Marine Biological Association of the United Kingdom90: 215-223
Wolkenhauer
S. (2008) Burying and feeding activity of adult Holothuria scabra (Echinodermata: Holothuroidea) in a controlled
environment. South Pacific Commission Beˆche-de-mer Information Bulletin 27,
25–28.
Yamanouchi,
T. (1939). Ecological and physiological studies on the holothurians in the
coral reef of Palao Islands. Palao Tropical Biological Station 25, 603-634.
|
|
|
|
|