|
|
|
Austrocochlea porcata (A.Adams, 1853)
|
|
Ali Adriana Copland 2018
|
|
|
|
Summary | |
Austrocochlea porcata is a top snail endemic to Australia, with a distribution ranging from Tasmania all the way to Queensland. A. porcata is an intertidal grazer that can be found on a range of substratum. There has been much contention as to the taxonomy and phylogeny of this species, just recently being classified as separate from its relative Austrocochlea constricta. A. porcata's taxonomy, life history, anatomy and physiology and ecology will be discussed below.
|
|
|
Physical Description | |
As part of the top-shell family (Trochidae), A. porcata has a characteristic conical shell shape. Adult shells can reach 34mm in length and 25mm in diameter (Parsons & Ward, 1994). Shells are thick and imperforate with a greenish-yellow periostracum (organic outer-layer, Figure 1) (Parsons & Ward, 1994). Most notably, the A. porcata shell is longitudinally striped on the spire and last whorl (Figure 2) (Parsons & Ward, 1994). Stripes alternate between parallel bands of off-white to black, or sometimes a dull red (Parsons & Ward, 1994), giving the species its zebra–like appearance. Parsons & Ward (1994) describe the spire of A. porcata as being “conical, acute, elevate or flattened”. Figure 2 demonstrates a somewhat flattened spire when compared to other species. The apex of the shell is often eroded, with some populations having a yellow internal colour (Figure 2) (Parsons & Ward, 1994). As seen in Figure 2, the apex can also have a silver looking colouration (personal observation) that also often becomes eroded as Parsons & Ward (1994) have documented, with more of a light-brown internal area, as opposed to yellow.
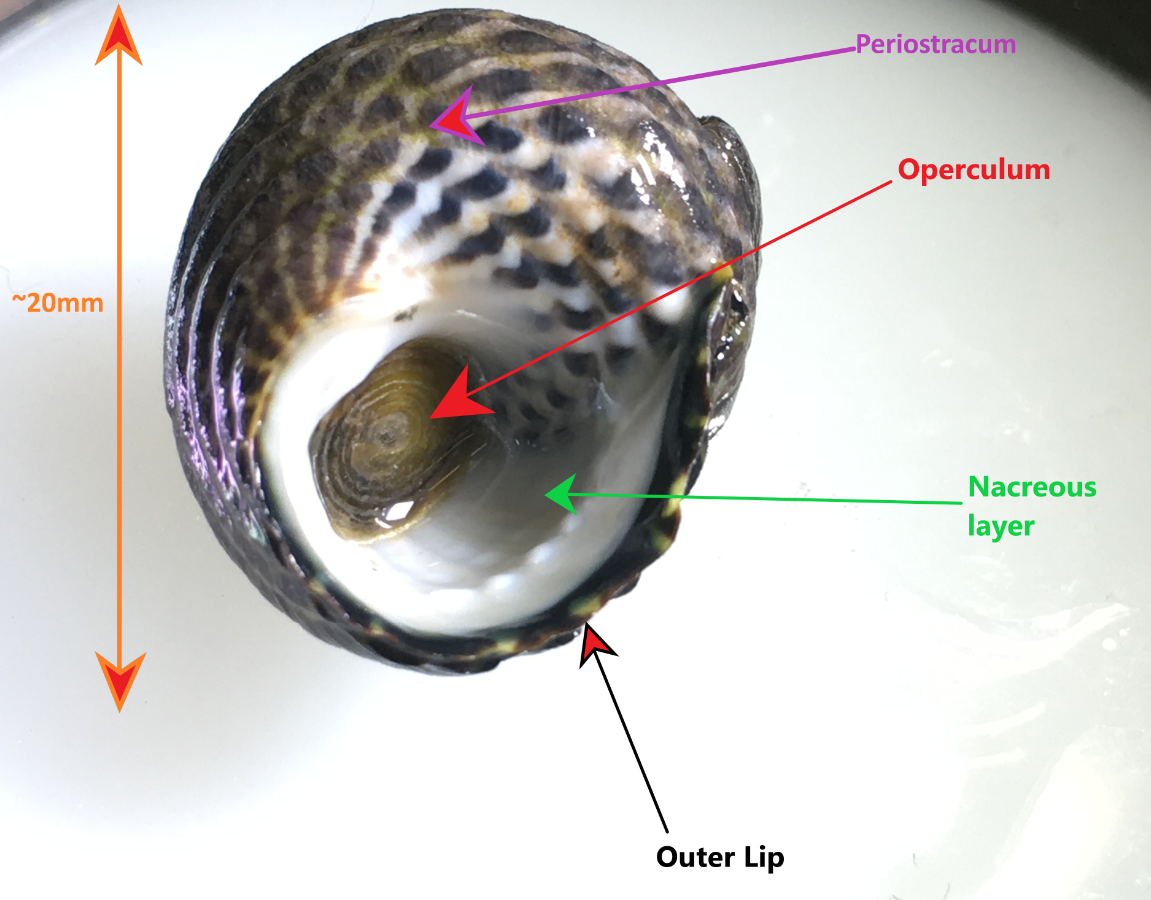
Figure 1: Specimen of A. porcata on its ventral surface, demonstrating the perisotracum, operculum, outer lip and inner nacreous layer. This specimen was collected in Wynnum, Queensland, Australia.
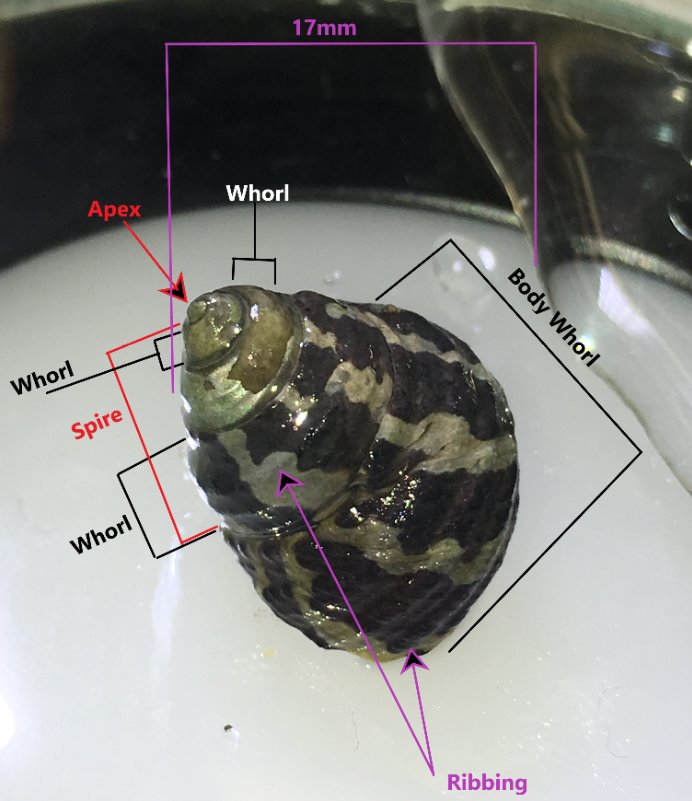
Figure 2: A. porcata specimen collected from Wellington Point, Queensland Australia. Components of the shell are marked such as the whorls, spire, apex and ribbing. This specimen possess a wide banding pattern and an eroded apex and first whorl. Four whorls are visible.
The teleconch (the entire shell excluding the tip of the apex) usually has 5-6 convex whorls on which the accremental stria run diagonally (Parsons & Ward, 1994). The shell is ribbed with the penultimate whorl usually having 3-4 ribs and the last whorl having 8-12 (Parsons & Ward, 1994). The outer lip of the shell is calcareous and striped, as well as slightly furrowed at the end of each rib (Figure 1) (Parsons & Ward, 1994). The inner nacreous layer of the shell is a pearly white colouration (Figure 1) (see ---> General Molluscan Body Plan). The operculum is circular and horny and has a somewhat transparent appearance (Figure 1), however, it may also look silver and reflective if placed under a bright light (personal observation) (Figure 3). The umbilicus of A. porcata is sealed, as seen in Figures 4 and 5 (Parsons & Ward, 1994).

Figure 3: A. porcata specimen from Wellington Point, Queensland, Australia. View of aperture/mouth of shell shows the operculum to be reflective under light, showing a silver appearance. Ventral view of snout and cephalic tentacles can also be seen.
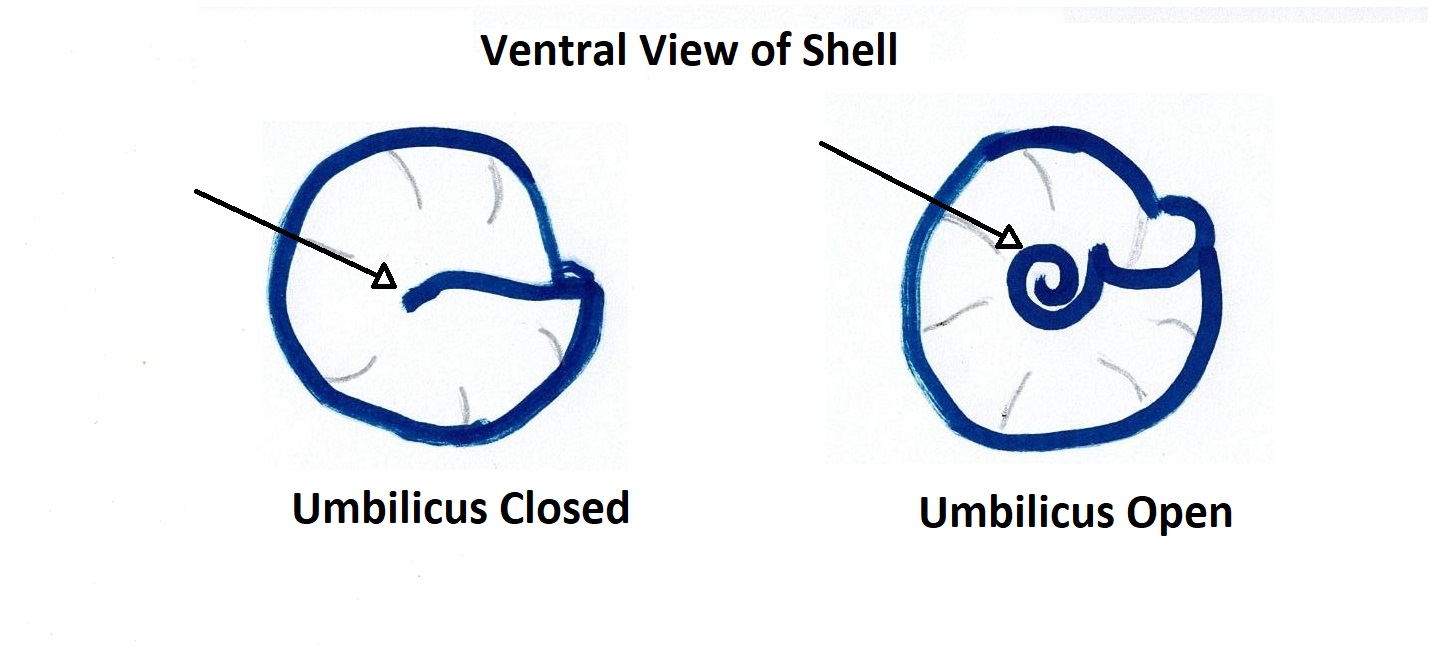 
Figure 4: Diagram illustrating the difference between an Figure 5: Ventral view of A. porcata specimen collected
open and closed umbilicus of a gastropod shell. from Wynnum, Queensland, Australia.
Picture demonstrates the closed umbilicus of A. porcata.
Parsons & Ward (1994) describe the head-foot as predominantly orange and black, with the foot being black with an orange band near the outer edge. However in specimens found in Queensland (Wellington Point) the ventral side of the foot was more of a buffish-green, while the ventral side of the foot was a black colour with a band of the buffish-green colour striped near the outer edge (personal observation ) (Video 1 & Figure 6). This similar colouration was also observed on the snout, however Parsons & Ward (1994) describe the snout colouration as having an orange stripe on a black snout. This difference in the orange/ green colours may be due to regional differences between Queensland and Tasmanian populations, where the majority of Parsons and Ward (1994) specimens came from. However, the cephalic lappets of the Queensland specimens seem to match the colouration described by Parsons & Ward (1994), which is green/orange and frondose, positioned anteriorly on the head (Figure 6).
Figure 6: Dissected head of A. porcata demonstrating the head, foot and cephalic lappets. Cephalic lappets have been cut down the middle and thus two halves can be seen in the photograph. Specimen was collected from Wellington Point, Queensland, Australia.
|
|
Video 1: Video of ventral view of A. porcata with head/foot protruding from shell.
Colouration of head/foot can be seen. |
Back to top of page
|
 |
| Figure 1 |
|
 |
| Figure 2 |
|
 |
| Figure 3 |
|
 |
| Figure 4 |
|
 |
| Figure 5 |
|
 |
| Figure 6 |
|
|
|
Ecology |
Habitat | |
A. porcata is a common trochid species endemic to Australia that occupies a relatively diverse range of habitats. A. porcata is commonly found in the mid-littoral zone of intertidal shores (Underwood, 1974; Creese & Underwood, 1976; Parsons & Ward, 1994), but also has estuarine representatives (MacPherson & Gabriel, 1962; Creese & Underwood, 1976). The type of substratum occupied by A. porcata within these habitats can vary greatly and includes: rocky, sandy, seagrass and mud-flat substratum (MacPherson & Gabriel, 1962; Parsons & Ward, 1994). Estuarine mud-flats, as well as seagrass and sandy substratum are usually well protected from wave-exposure (MacPherson & Gabriel, 1962; Parsons & Ward, 1994). A. porcata can still however thrive in exposed areas such as the rocky intertidal (Parsons & Ward, 1994).
|
 |
| Figure 7 |
|
 |
| Figure 8 |
|
|
|
Diet | |
With use of their specialised
radula (see ---> Physiology of A. porcata), A. Porcata grazes on diatoms
and algal spores (Creese & Underwood, 1976).
|
|
|
Relationships | |
A. porcata coexist with many other
gastropod grazers of the intertidal zone, such as limpets or other top shells. A. porcata is found in sympatric
populations with its sister species, A.
constricta (Figure 9). Furthermore, A. porcata has actually been observed to
have a commensal relationship with the limpet, Patelloida mufria (Mapstone, Underwood & Creese, 1984). P. mufria were found almost exclusively
on the shell of A. porcata in the
intertidal zone (Mapstone et al., 1984). They were most common on A. porcata that spent low tides in rock
pools and were observed to always inhabit the underside of the snail’s shell
when out of a rock pool (Mapstone et al., 1984). When out of rock pools and separated
from hosts, the limpets would more quickly succumb to desiccation or be
predated upon by predatory whelks (Mapstone et al., 1984). When on a host,
predation by whelks was virtually reduced to zero, most likely due to A. porcata’s ability to avoid predators (Fairweather
& Underwood, 1983). It is also
interesting to note that P. mufria preferentially
chose hosts that had not previously been grazed by a conspecific limpet and were less
likely to choose A. porcata that had algae
removed (Mapstone et al., 1984). Thus, the benefits of a commensal relationship
with A. porcata include an increased
chance of being submerged at low-tide, decreased risk of predation and
potentially increased food supply (Mapstone et al., 1984). It
is also worth noting that this study refers to A. porcata’s sister species, A.
constricta, however, it has since been suggested that the study was really
conducted on A. porcata (see Evolution and Systematics )
(Beesley, Ross & Wells, 1998).

Figure 9: Image of both A. porcata and A. constricta in a sympatric population at Flat Rock Point, New South Wales, Australia. Image courtesy of Dr Don Colgan.
As for predators of A. porcata, Chilton & Bull (1984)
investigated the influence of predation by the reef crab Ozius truncates on a closely related species, Austrocochlea concamerata.
O. truncates is also known to prey
on other intertidal gastropods and has a distribution that coincides with A.
porcata, thus it is reasonable to assume that O. truncates would
predate on A. porcata. In fact, Coleman et al. (2014) also assumed this
and used A. porcata in an experiment investigating shell repair when simulating
damage similar to that caused by durophagous (shell crushing) crabs (see Conservation and Threats).
Back to top of page
|
 |
| Figure 9 |
|
|
|
|
Life History and Behaviour | |
Most gastropods have two different larval stages, the trochophore larval stage and the veliger larval stage. The trochophore (Figure 10A) is a characteristic larval stage of molluscs and is the hatching stage of some primitive mollusc clades (Ruppert et al., 2004). Other clades have suppressed the trochophore stage so that it occurs within the egg, and instead the hatching stage is a veliger larvae (Ruppert et al., 2004). The veliger larval stage (Figure 10B) is derived from a trochophore stage but is more developed, occurring later in development (Ruppert et al., 2004). Veliger larva are characteristic of the gastropods, bivalves and scaphopods (Ruppert et al., 2004). An important aspect of the veliger larvae is its swimming organ, the vellum, which consists of two semi-circular ciliated lobes (Figure 10B) (Ruppert et al., 2004). Veliger larvae can be both feeding/ planktonotrophic, or non-feeding/ lecithotrophic (yolk-laden) (Ruppert et al., 2004).
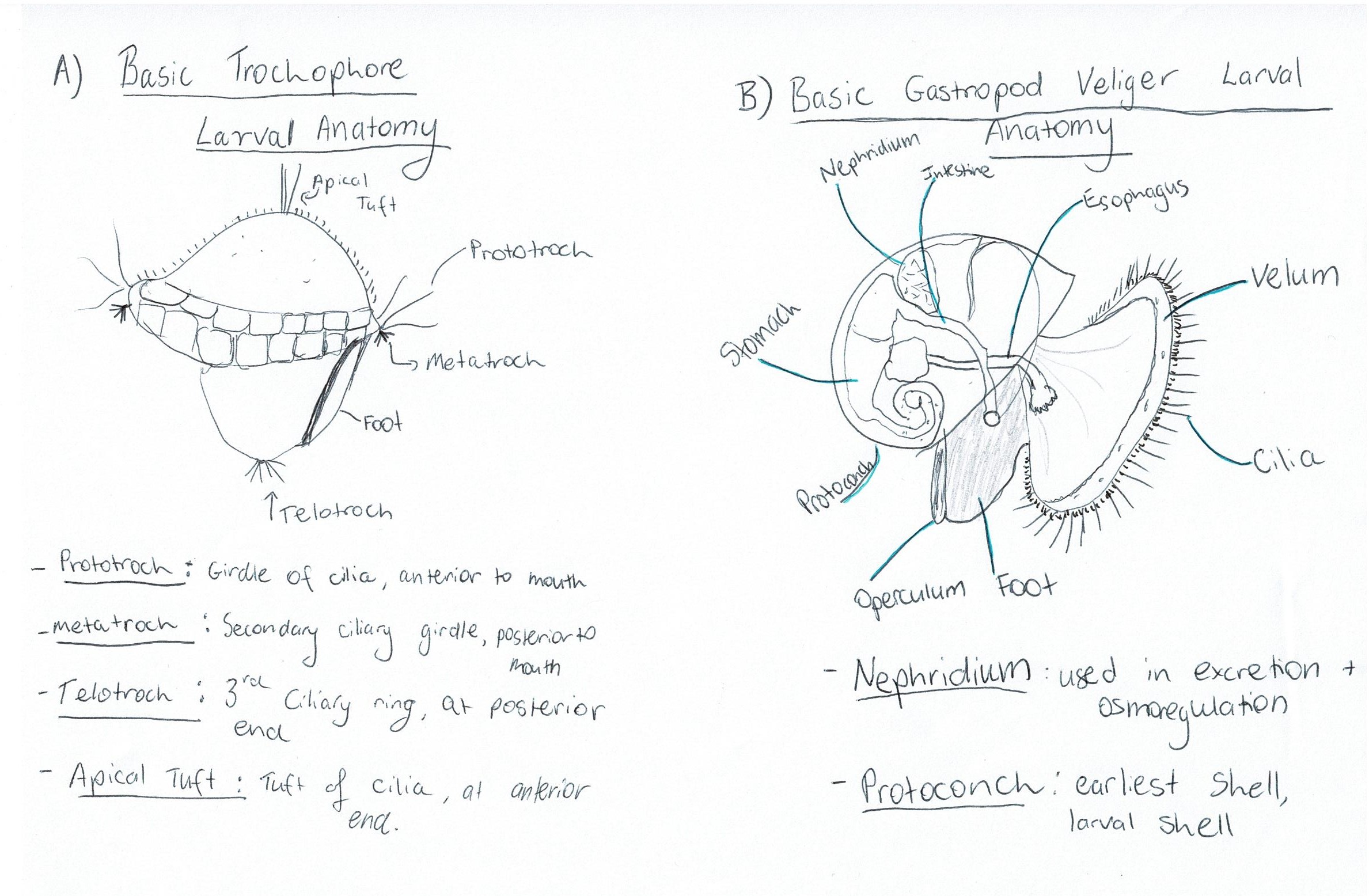
Figure 10: Diagrams of: A) basic trochophore larval anatomy and B) Basic veliger larval anatomy of a gastropod, with some important terms defined.
Unfortunately the exact life
history of A. Porcata has yet to be described. Nonetheless, inferences can be
made from closely related species. A.
Porcata is a dioecious species, meaning that males and females are two separate individuals. Underwood (1974) studied A.
Constricta and noted like many other gastropods, A. constricta had external
fertilisation, meaning they would release eggs and sperm into the environment and not develop the juveniles within the female. It is not known whether the species would release eggs and sperm directly into water, or if eggs were laid in ribbons or masses on substratum, as in other species. However, Underwood (1974) noted that A. constricta interestingly would breed continuously throughout the year and characterised the spawning as "broadcast spawning", which indicates eggs are deposited directly into the ocean. This is somewhat unusual as most broadcast fertilising species maximise
fertilisation by having a restricted and synchronised breeding season (Giese,
1959). Underwood (1974) also suggests that A. constricta is
likely to have a similar lecithotrophic larval stage as another closely related
Trochid, Gibbula cineraria, an
assumption which is shared by later studies as well (Colgan & Schreiter,
2010). This is also somewhat unusual as most broadcast spawning archaeogastropods are known to have free-swimming trochophore larvae, not lecithotrophic larvae (Ruppert, Fox & Barnes, 2004). Nonetheless, due to the close relatedness of both A. constricta and G. cineraria to A. porcata, as well as the fact that it is quite likely Underwood's (1974) study may have been conducted on A. porcata due to differences in nomenclature at the time (see Evolution and Systematics), it is assumed that their life histories are synonymous with A. porcata.
Underwood (1972) investigated
this lifecycle, particularly the larval stages. Upon fertilisation, spiral
cleavage of the embryo was observed. Following gastrulation, formation of a
trochophore larvae could be seen (Figure 11, Stage 1) (Underwood, 1972). 11-12 hours post
fertilisation, trochoblasts became ciliated and the prototroch was clearly
visible by 16 hours post fertilisation (Underwood, 1972). By this stage the
cilia were also well developed and the blastopore was completely closed
(Underwood, 1972). At 19 hours, the trochophore larvae is nearly fully
developed within the egg membrane and the ciliary beat is more controlled but
continuous, with the larva beginning to rotate within the egg (Figure 11, Stage 1) (Underwood, 197).
By 24 hours, neural coordination of the larva is observed as the
trochophore is able to stop and start the ciliary beat (Underwood, 1972). At
approximately 28 hours hatching occurred, involving the gradual disintegration
of the egg membrane and violent movements of the protrochal cilia (Underwood,
1972)
After hatching, the animal is at
the veliger larval stage (Figure 10B, Figure 11 Stage 2). Shell formation was observed immediately after
hatching via eversion of the shell gland, which resulted in a thin patch of
cells on the trochophore's surface (Underwood, 1972). These cells would
eventually give rise to a cup-shaped transparent shell (Underwood, 1972). This
stage also sees the foot beginning to form (Underwood, 1972). At around 33
hours post fertilisation, the velar cells began to migrate to the anterior of
the larva to form the vellum (Underwood, 1972). The mantle fold and mantle
cavity were also formed at this stage (Figure 11 Stage 2) (Underwood, 1972). At approximately 40
hours, the foundations of the operculum were visible on the rudimentary foot
and the digestive gland was seen to differentiate (Underwood,
1972). 40-44 hours post fertilisation was the stage immediately before
torsion (Figure 11 Stage 2)( for Torsion see ---> The Makings of a Gastropod) where the retractor muscle is also developed (Underwood, 1972).
Before torsion occurred, the lava
exhibited an interesting behaviour, whereby the swam to the water’s surface and
then would cease swimming and sink (Underwood, 1972). The first step in torsion
then took place at 48 hours, with a 90° rotation
resulting in the mantle cavity being on the right hand side, and the foot and velum also being rotated (Figure 11 Stages 3 & 4) (Underwood, 1972). The second 90° rotation took place in the next 4 days with the larva
now able to partially retract into the shell (Figure 12 Stage 5) (Underwood, 1972). Following this stage, the larvae continue to
develop for 4-5 days after torsion, at which stage they can move the shell
upright and crawl with the sole of the foot (Figure 12 Stage 6) (Underwood, 1972). The crawling juvenile will then settle and continue to grow into an adult (Figure 12 Stage 7).
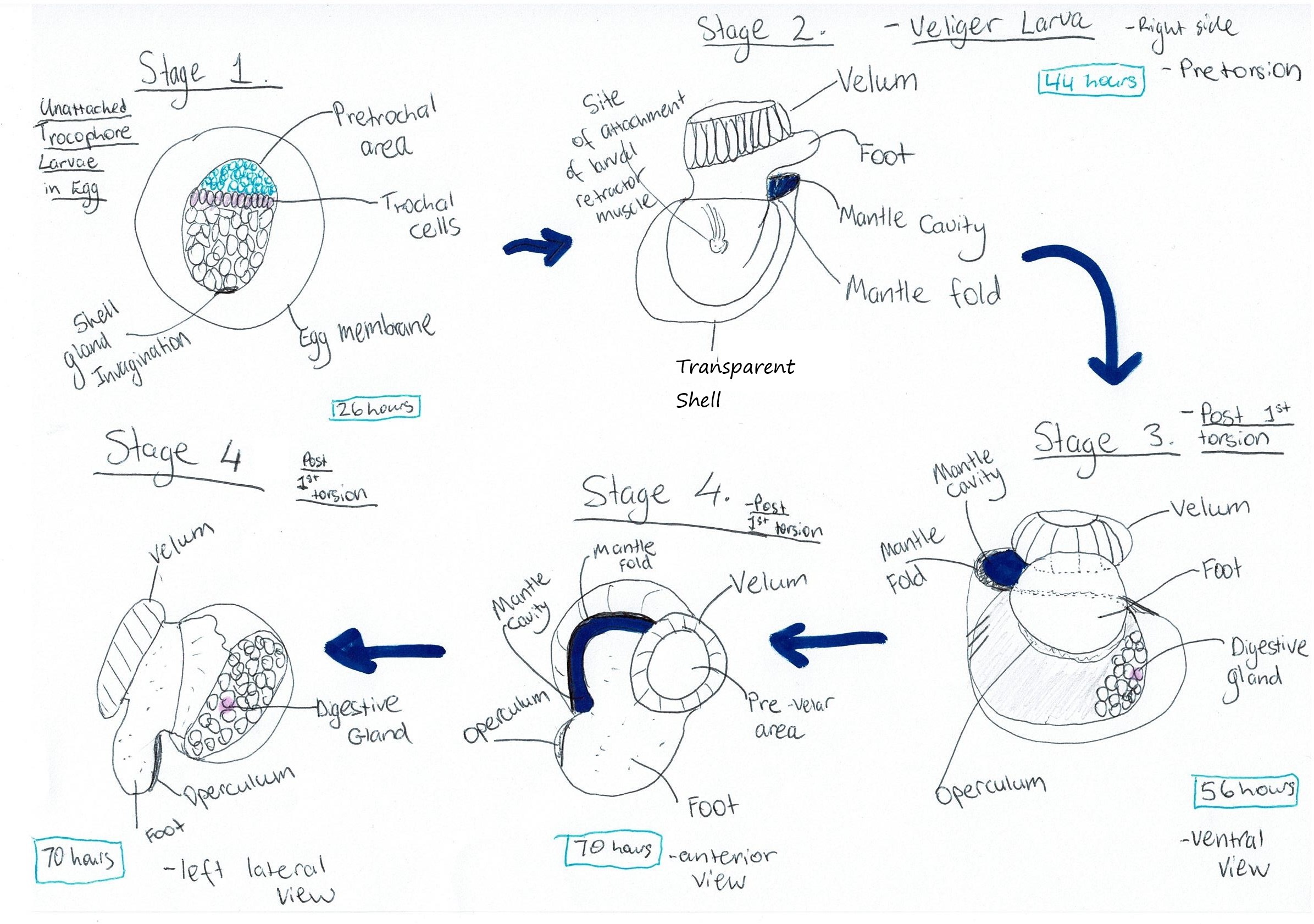 Figure 11: Diagram of A. porcata's larval developmental process. Cilia are omitted from the diagrams. Adapted from Underwood (1972), "Spawning, Larval Development and Settlement Behaviour of Gibbula cineraria (Gastropoda: Prosobranchia) with a Reappraisal of Torsion in Gastropods."
Figure 11: Diagram of A. porcata's larval developmental process. Cilia are omitted from the diagrams. Adapted from Underwood (1972), "Spawning, Larval Development and Settlement Behaviour of Gibbula cineraria (Gastropoda: Prosobranchia) with a Reappraisal of Torsion in Gastropods."
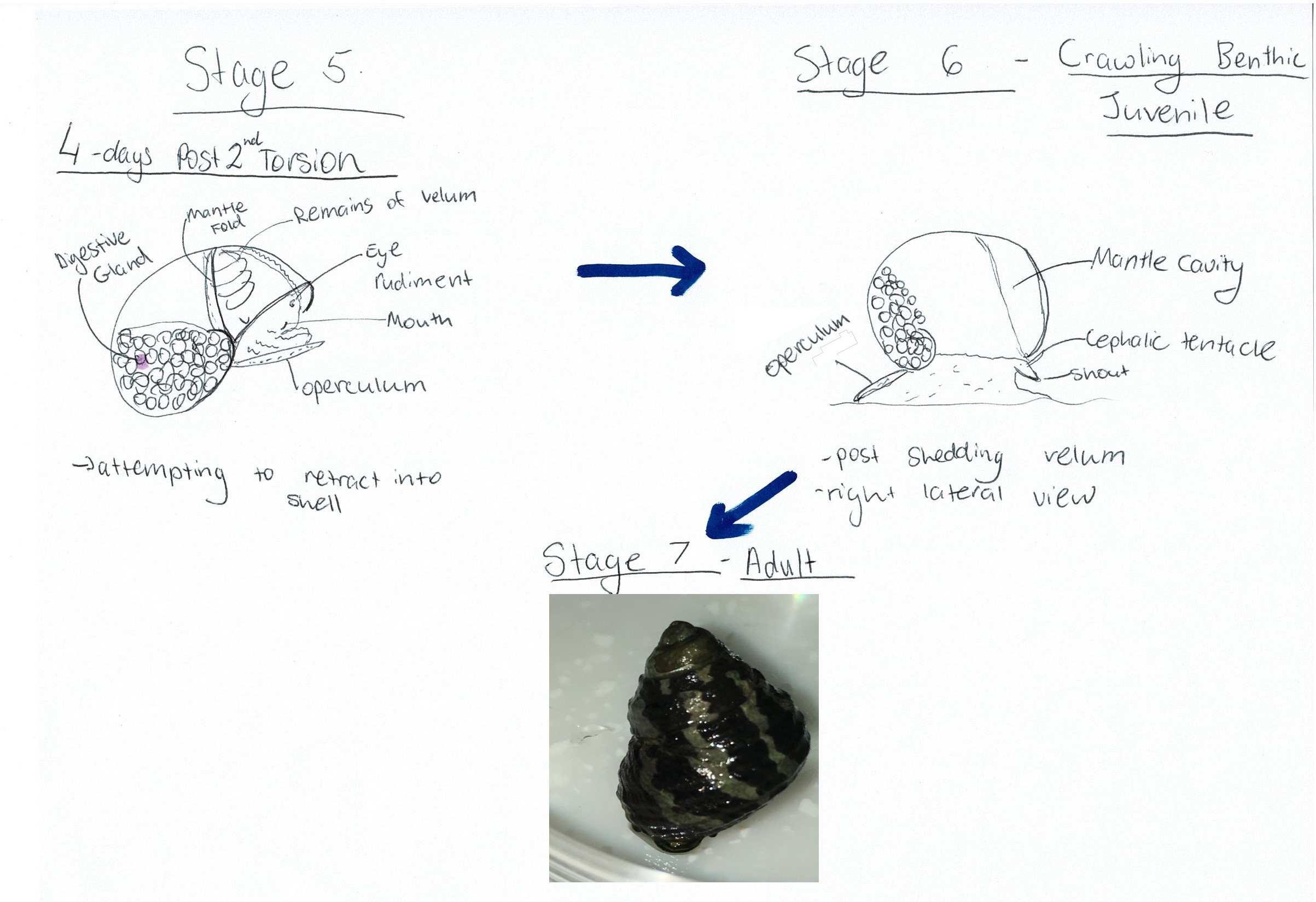 Figure 12: Diagram of A. porcata's developmental process after torsion has been completed. Adapted from Underwood (1972), "Spawning, Larval Development and Settlement Behaviour of Gibbula cineraria
(Gastropoda: Prosobranchia) with a Reappraisal of Torsion in Gastropods."
Back to top of page
Figure 12: Diagram of A. porcata's developmental process after torsion has been completed. Adapted from Underwood (1972), "Spawning, Larval Development and Settlement Behaviour of Gibbula cineraria
(Gastropoda: Prosobranchia) with a Reappraisal of Torsion in Gastropods."
Back to top of page
|
 |
| Figure 10 |
|
 |
| Figure 11 |
|
 |
| Figure 12 |
|
|
|
Anatomy and Physiology |
General Molluscan Body Plan | |
Mollusca is one of the most diverse,
yet well-known phyla in existence. With well over 90000 described living
mollusc species (Brusca & Brusca, 2003), there comes a great diversity in
molluscan body plans. However, there are several key defining features that are
common to all molluscs.
Firstly, all molluscs are
protostomes (Brusca, Moore & Shuster, 2016). The word protostome comes from
two Greek words; πρωτο (prōto) meaning “first” and στόμα (stoma) meaning
“mouth”. “First mouth” is used to describe the embryonic origin of the mouth
and to distinguish protostomes from their counterparts the deuterostomes
(second mouth) (Pechenick, 2010). In embryonic development, the mouth of a
protostome develops from the blastopore, which is the first opening formed in
the embryo (Pechenick, 2010). However, in deuterostomes, the anus is formed
from the blastopore and the mouth is developed secondarily from another
opening, thus the name “second mouth”.
All molluscs are also
unsegmented, coelomates (Brusca et al., 2016). Coeloms are fluid-filled body
cavities that are lined by mesothelium (middle epithelium) (Figure 13). Coeloms
are a unique innovation of the bilaterians, which are a group of bilaterally
symmetrical animals that Mollusca are a part of (Ruppert et al,
2004). Coeloms provide a third body compartment that can be used as a
hydrostatic skeleton or for physiological roles such as excretion, reproduction
and circulation (Ruppert et al., 2004). The molluscan coelom has become limited
to small spaces in the nephridia (like kidney), heart and gonads (Brusca et al., 2016) (Figure 14). This reduction in
the coelom often coincides with connective tissue developing into a hemal
system (Ruppert et al., 2004). Hemal systems carry blood through interconnected
vessels and cavities, such as sinuses (Ruppert et al., 2004). In the case of
molluscs, a hemocoel is present, which is the product of a large hemal sinus
becoming the principal body cavity (Ruppert et al., 2004). This means that
molluscs also possess an open circulatory system (Brusca et al., 2016).
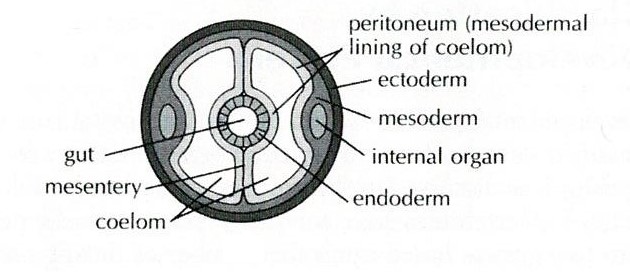 Figure 13: Diagram of a coelom. Sourced from: Pechenick (2010), Biology of the Invertebrates.
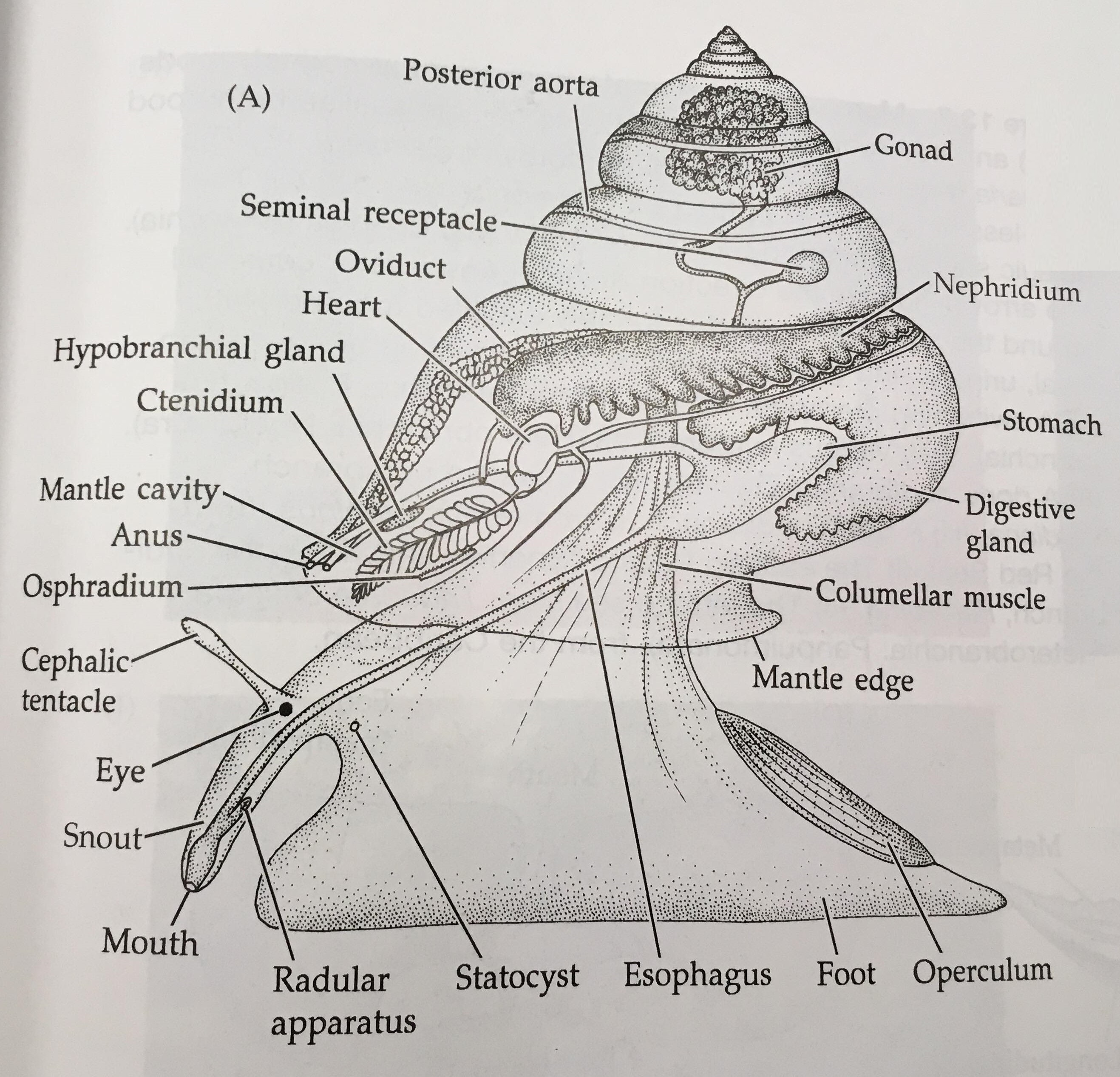
Figure 14: Key features of a gastropod mollusc. Image sourced from Brusca & Brusca (2003), Invertebrates (second edition).
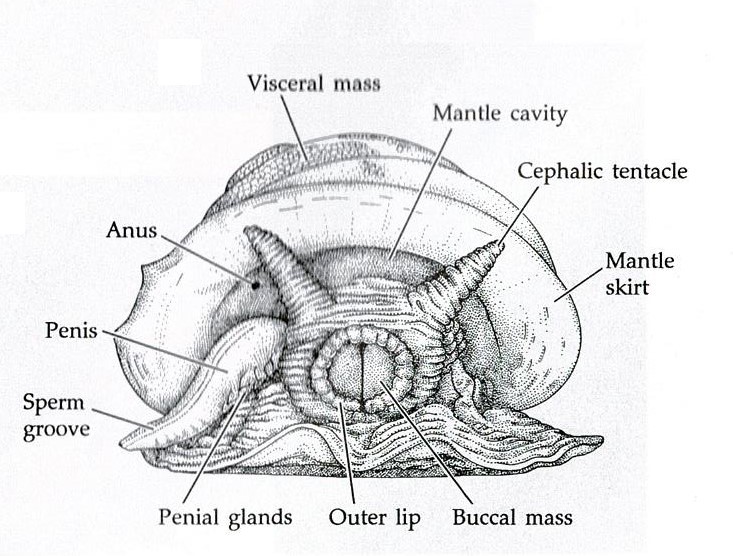
The body of a mollusc is made up of three general regions; the head, foot and visceral mass (Brusca & Brusca, 2003). The visceral mass (mass of internal organs) is located dorsally with the muscular foot located ventrally, as seen in Figure 14 (Brusca & Brusca, 2003). The head may house sensory organs such as eyes and tentacles (Brusca & Brusca, 2003), which are of particular importance to mobile molluscans such as cephalopods and gastropods. The molluscan body itself is enclosed in a thick cuticle-covered epidermal sheet of skin known as the mantle (Brusca et al., 2016). The mantle overhangs and forms the mantle cavity (Figures 14 & 15) which houses various important organs such as the ctendia or gills, anus and gonopores (Brusca et al., 2016).
The mantle also plays an
important role in regards to organisation of the body. Shell glands within the
mantle secrete sclerites or plates that will be housed in the body wall. These
sclerites or plates form the hard, calcareous skeleton of some molluscs (Brusca
& Brusca, 2003). Conversely, a solid shell can also be produced by the
mantle, which can be internal or external depending on the class (Brusca &
Brusca, 2003). To construct the shell, calcium carbonate is produced
extracellularly and deposited in layers (Figure 16). (Brusca & Brusca, 2003). However, the outermost surface layer of the shell consists of a thin organic
coating called the periostracum (Brusca & Brusca, 2003). The periostracum
is made up of a type of protein called conchin, which is similar to the
proteins found in the epidermal cuticle (Brusca & Brusca, 2003). The two
thicker calcareous layers are found underneath the periostracum. Immediately
under the periostracum is the prismatic layer, of a chalkier appearance (Brusca
& Brusca, 2003). The lamellar, or nacreous layer, is the inner most
calcareous layer and has a pearly appearance (Brusca & Brusca, 2003). Both
the prismatic and nacreous layers incorporate conchin like the periostracum,
this aids in binding the calcareous crystals together (Brusca & Brusca,
2003). The nacreous layer has been lost in many groups but is present in gastropods
such as A. porcata (Figures 17 & 18).
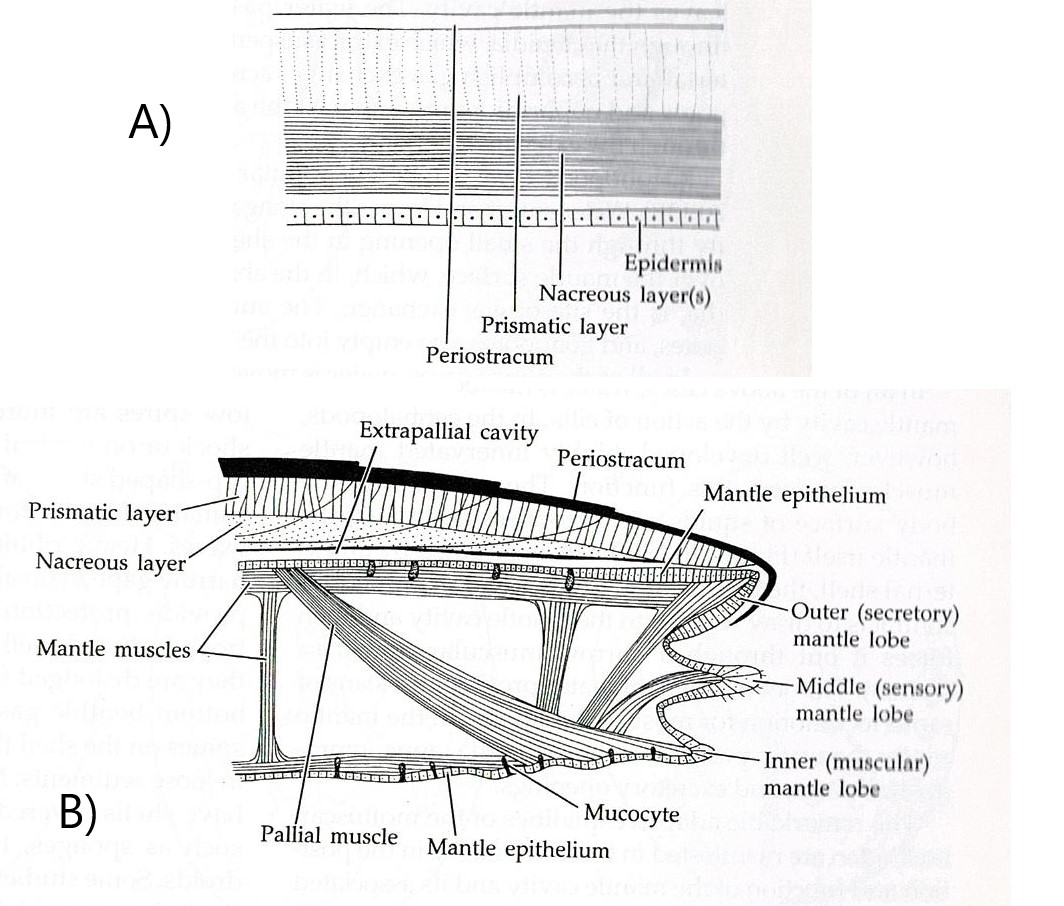
Figure 16: Diagram representing the different layers of a molluscan shell. Image sourced from Brusca & Brusca (2003), Invertebrates (second edition).
Figure 17: Fragment of A. porcata shell showing the Figure 18: Fragment of A. porcata shell showing the three shell layers. three shell layers.
Molluscs also possess a complete digestive
system (mouth and anus) that has regional specialisation (Brusca et al., 2016).
One structure that is unique to molluscs is the radula, located in the buccal
region of the foregut (Brusca & Brusca, 2003). The radula is a toothed,
tongue-like apparatus used to feed in a rasping manner (Brusca & Brusca,
2003).
Back to top of page
|
 |
| Figure 13 |
|
 |
| Figure 14 |
|
 |
| Figure 15 |
|
 |
| Figure 16 |
|
 |
| Figure 17 |
|
 |
| Figure 18 |
|
|
|
The Makings of a Gastropod | |
Torsion and Shell Spiraling
As well as sharing all of the
basic molluscan characteristics mentioned above, gastropods possess some
innovations that are unique to their class, namely torsion and shell spiraling.
Torsion is a unique synapomorphy
that takes place in gastropod development, usually in the late veliger larval
stage (see Life History and Behaviour) (Brusca & Brusca, 2003). Torsion is a
counter-clockwise rotation of the visceral mass, along with its overlaying
mantle and shell, of sometimes up to 180° in regards to the head and foot (Brusca
& Brusca, 2003). Torsion is usually a two-step process resulting in the
mantle cavity and anus moving from a posterior position to an anterior
position, while the viscera and developing organs that were on the right side
of the larval animal move to the left of the adult (Brusca & Brusca, 2003).
The first step in this process is
the initial 90° rotation of the shell and viscera. This rotation is caused by
contraction of the velar/foot retractor muscle (Figure 19 (E)) that forms during
larval development (Brusca & Brusca, 2003). The muscle attaches to the right side of the
shell and extends over the gut, then attaches to the left side of the head and
foot (Figure 19 (E)) (Brusca & Brusca, 2003). When this muscle contracts, the shell
and associated viscera rotate counter-clockwise by 90° (Brusca & Brusca, 2003).
This twist is much quicker than the second subsequent 90° rotation, taking place in as
short as minutes or up to a few hours (Brusca & Brusca, 2003). The second
step in torsion is another 90° rotation, resulting in 180°
movement of the visceral mass and shell. The second rotation happens much
slower than the first and is not the result of muscle contraction (Brusca &
Brusca, 2003). Instead, this rotation is a result of differential tissue growth
(Figure 18 (B, C, D)) (Brusca & Brusca, 2003). Some gastropods retain torsion into
adulthood while other become partially or fully detorted (Brusca & Brusca,
2003).
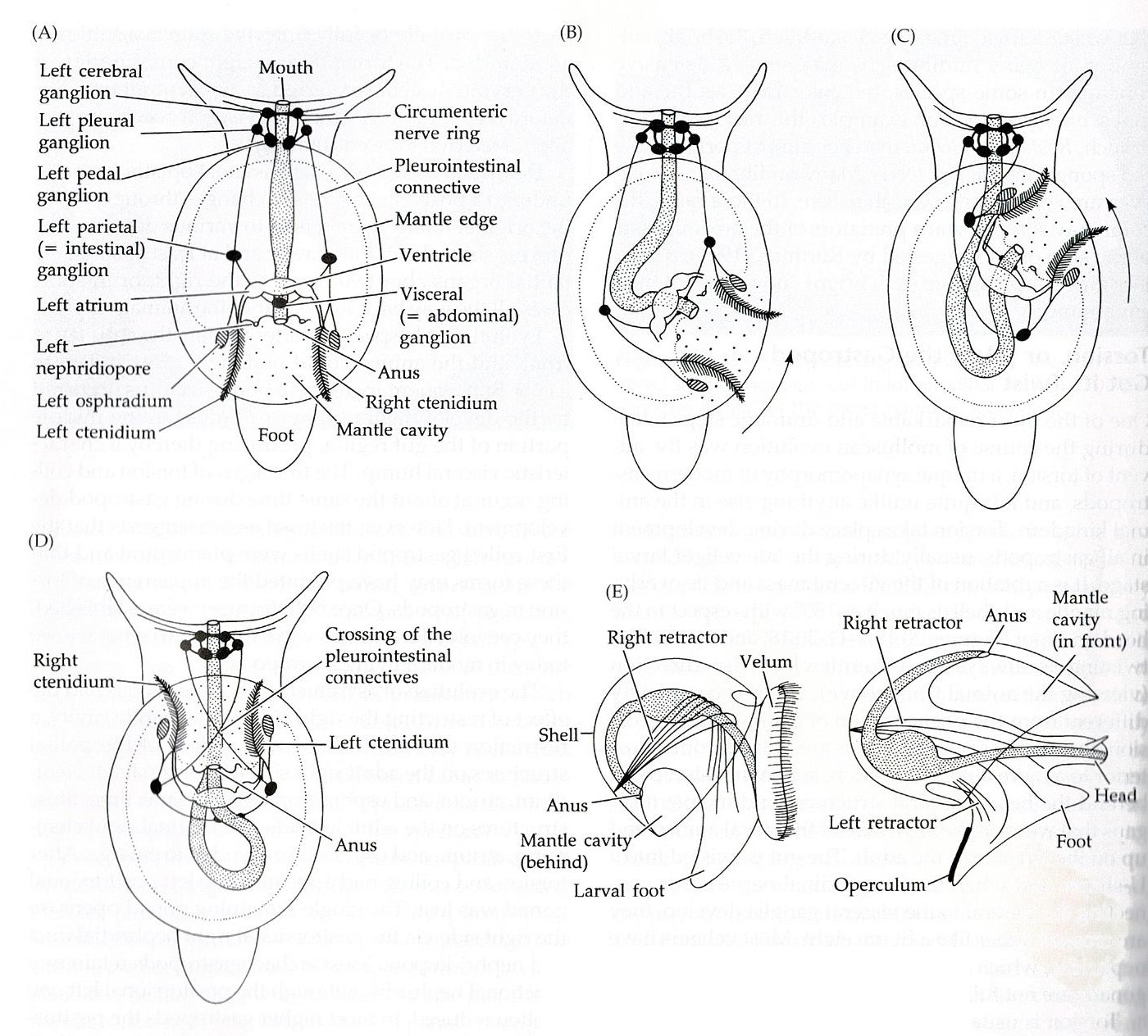
Figure 19: A) Diagram of theoretical pre-torted gastropod; B & C) Demonstration of torsion process; D) Diagram of post-torsion gastropod; E) Demonstrates placement of retractor muscle. Image sourced from Brusca & Brusca (2003), Invertebrates (second edition).
Shell spiralling is a separate process
that arose to accommodate a larger visceral mass in a monoplacophoran ancestor,
as well as to allow for a space to retreat into (Ruppert et al., 2004). All whorls of the first coiled shells were in
a single plane, planispiral, and the animal was thus bilaterally symmetrical (Ruppert
et al., 2004). However, problems began to arise due to the large, disc-shaped
nature of a planispiral shell and the fact that each whorl of a planispiral
shell lays outside the circumference of the preceding one (Ruppert et al.,
2004). This meant that small increases in biomass required large increases in
shell diameter, resulting in shells with a very high centre of gravity (Ruppert
et al., 2004). This issue was solved by asymmetrical conispiralling. Conispiralled
shells spiral out to the side, usually the right, and have whorls that overlap
and enclose the preceding whorl (Ruppert et al., 2004). To bring the centre of
gravity of the shell over the visceral mass and not to the right, the body axis
also changes (Ruppert et al., 2004).
Back to top of page
|
 |
| Figure 19 |
|
|
|
Physiology of A. porcata | |
Locomotion
Locomotion in A. porcata is achieved by the muscular gastropod foot which is comprised of muscle and connective tissue, as well as flat creeping sole (Ruppert et al., 2004). Two of the most important muscles for locomotion are the columellar, on the dorsal and central areas of the foot, and tarsos, on the ventral and outer edges of the foot (Ruppert et al., 2004). The columellar is made up of large muscle bundles that are encased in connective tissue (Ruppert et al., 2004). These bundles function as hydrostatic skeletons and are able to antagonise each other, thus enabling extension and retraction of the foot to occur (Video 2) (Ruppert et al., 2004). As well as being responsible for extension and retraction of the foot and head, the columellar muscle complex can also twist the foot with respect to the shell (Ruppert et al., 2004). The tarsos is made up of much smaller muscle fibres and functions as the sole of the foot, thus being involved in locomotion, as well as other things such as prey capture. The sole of the foot is laden with many secretory glands that produce mucus over which the animal glides (Ruppert et al., 2004). In order to move, A. porcata propels waves of muscular contraction that move along the sole of the foot (Video 3) (Ruppert et al., 2004). The sticky mucus enables the sole of the foot to grip the substratum and when in a wave the mucus becomes liquiefied, permitting the foot to slide forwards (Ruppert et al., 2004).
|
Video 2: A. porcata protruding from overturned shell. Extension and retraction of the foot/snout is visible. Eyes and cephalic tentacles can also be seen.
|
Miller (1974) investigated the mode of locomotion for numerous prosobranch gastropods. Some Trochidae species were investigated and found to exhibit both direct and retrograde ditaxic wave patterns (Figure 20) (Miller, 1974). A ditaxic wave pattern refers to “a separate set of waves passing alternately over the right and left sides of the sole of the foot” (Miller, 1974), as opposed to one wave that spans the entire foot. Direct waves are those that start at the posterior of the animal and move towards the front, thus travelling in the same direction as the animal itself (Miller, 1974). Retrograde waves are the opposite of direct waves, these waves start at the anterior of the animal and move to the back, thus moving in the opposite direction of travel (Miller, 1974). No representatives from the Austrocochlea genus were represented in the study thus it is not confirmed whether A. porcata uses direct or retrograde waves of muscle contraction. However, after analysing a recording of an A. porcata foot it seems that direct ditaxic waves of muscle contraction were used, as waves seemed to move from posterior to anterior (Video 3).
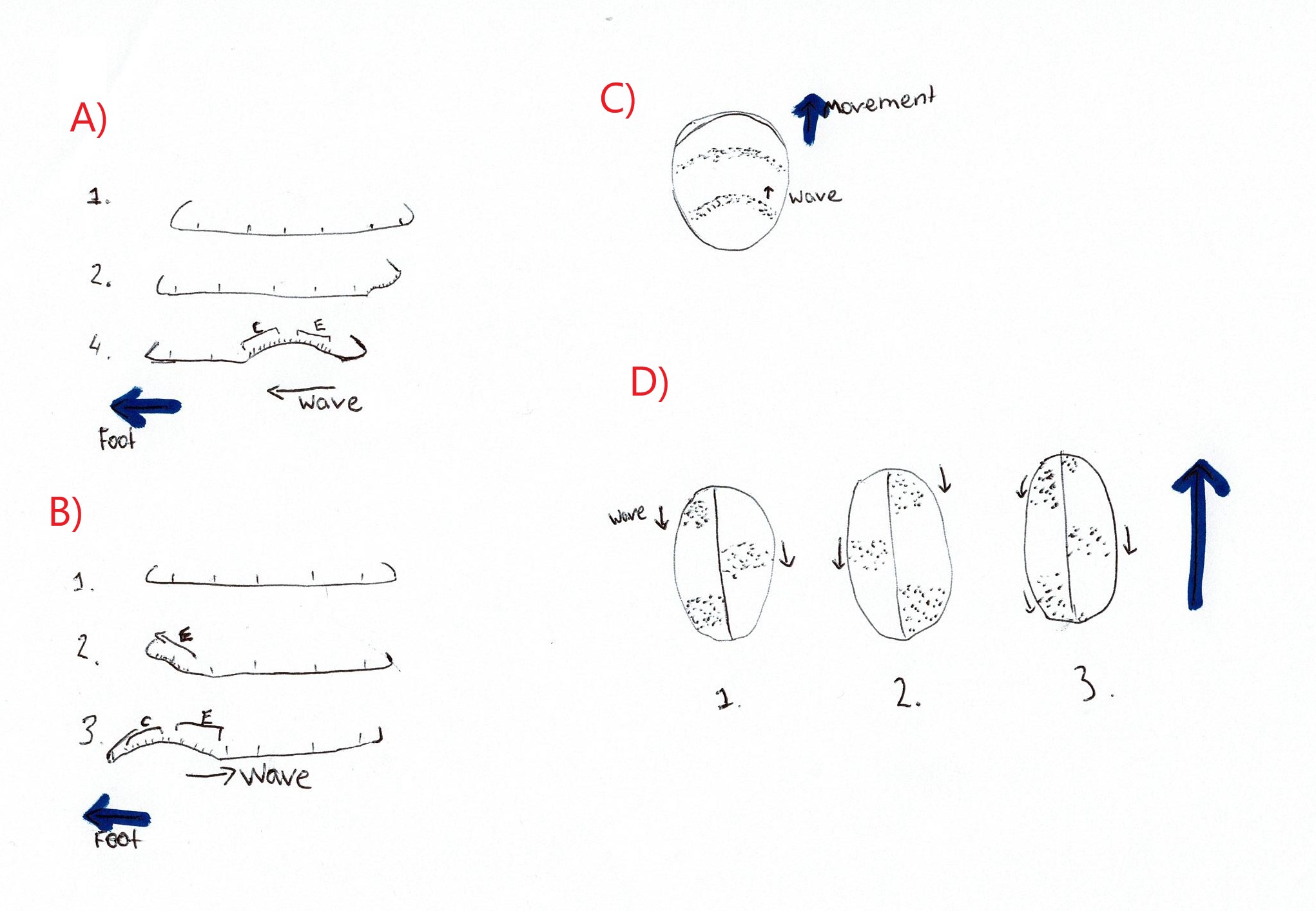
|
Video 3: Ventral view of the zebra top shell A. Porcata. Muscular contractions are in the form of ditaxic direct waves.
|
Digestion and Feeding
The digestive system of A. porcata includes a mouth, buccal cavity, oesophagus, stomach, intestine, rectum and anus (Ruppert et al., 2004). Food items are collected by the specialised radula and mixed with mucus (Ruppert et al., 2004). This mucus mix is pulled through the esophagus and into the stomach by a rotating protostyle (Figure 21). The stomach is the site of extracellular digestion which occurs using enzymes from the oesophageal pouches and digestive cecum (Ruppert et al., 2004). The digestive ceca (Figure 21) have multiple functions including the ability to secrete enzymes, phagocytose particles for intracellular digestion, and to absorb digested molecules for transport to the hemocoel (Ruppert et al., 2004). The intestine runs from the anterior end of the stomach and opens via the anus on the right side of the mantle cavity (Figure 21) (Ruppert et al., 2004). It is involved in the formation, packaging and storage of feces (Ruppert et al., 2004).
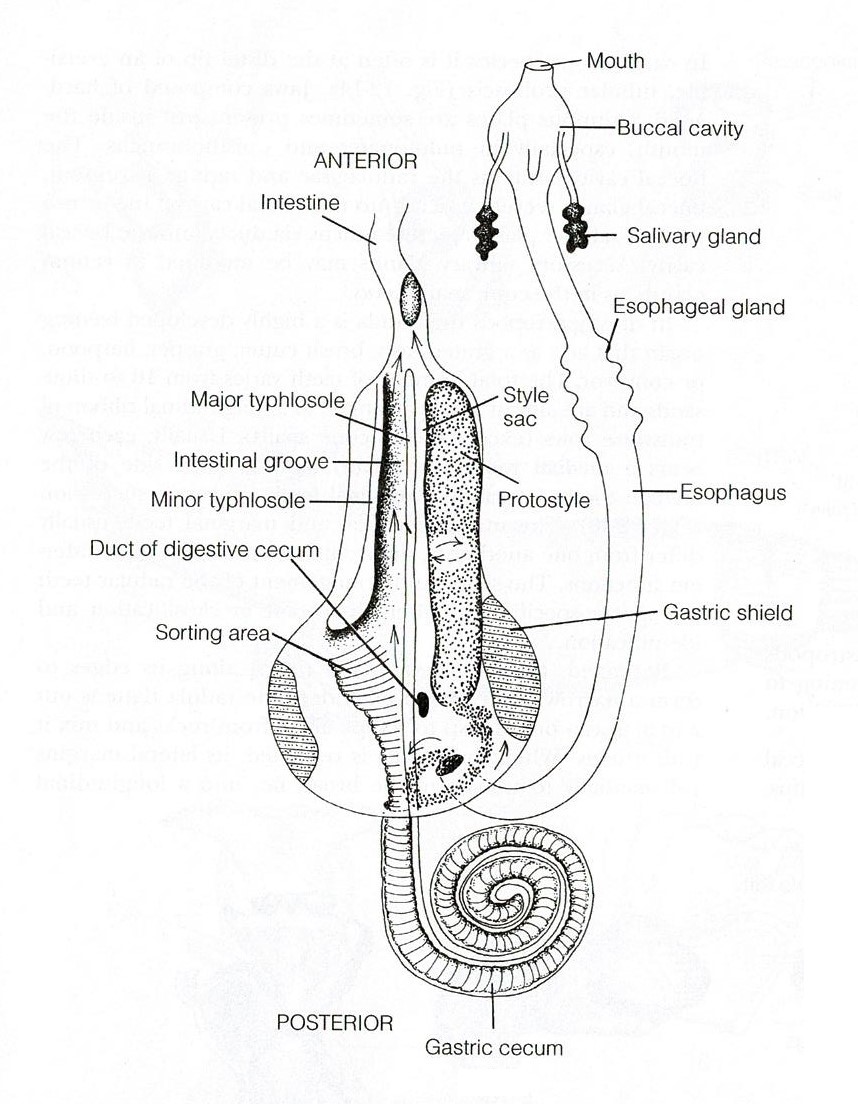
Figure 21: Digestive tract of a typical archaeogastropod, Trochidae. Image sourced from Ruppert, Fox & Barnes (2004), Invertebrate Zoology (Seventh Edition)
In order to gather food to digest, A. porcata uses its specialised radula. The raudula of archaeogastropods, such as A. porcata, is rhipidioglossate, which means fan-like (Ruppert et al., 2004). Each row of a rhipidioglossate radula has an abundance of teeth, split into three types; marginal, lateral and median (rachidian) (Figure 22 & 23) (Ruppert et al., 2004). However, this structure of a radula is not common to all gastropods, radulae are extremely diverse and are closely tied to an organism's life-history and diet.
Within the A. porcatas subfamily Monodontinae (also known as Trochinae) there is still a large diversity of radulae, but all are characterised by some key features. These include, the central and marginal tooth fields being well developed and forming acute rows (Beesley et al., 1998). Within these rows the tooth bases and cusps coincide (Beesley et al., 1998). The rachidian is flanked by five pairs of lateral teeth with overhanging cusps (Beesley et al., 1998). Both the rachidian tooth and lateral teeth have a base that is broad, but narrows towards the cusp, creating a shaft (Figure 24). The base and shaft of the innermost marginal tooth is fused and enlarged and attaches to the cusp (Beesley et al., 1998).
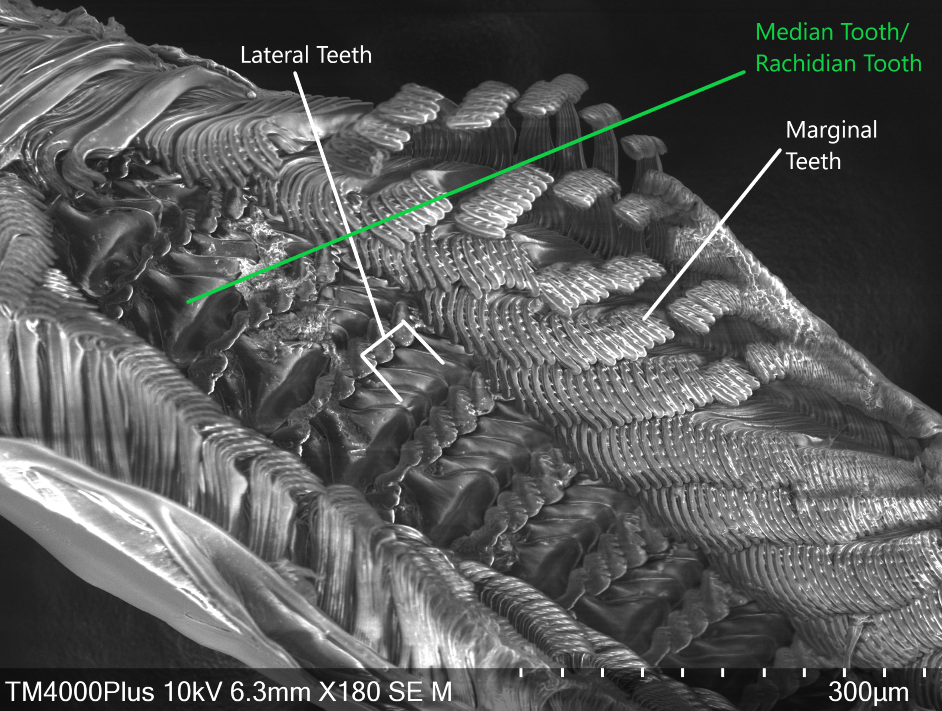
Figure 22: Scanning Electron Microscope image of A. porcata radula. Marginal, lateral and median teeth are noted.
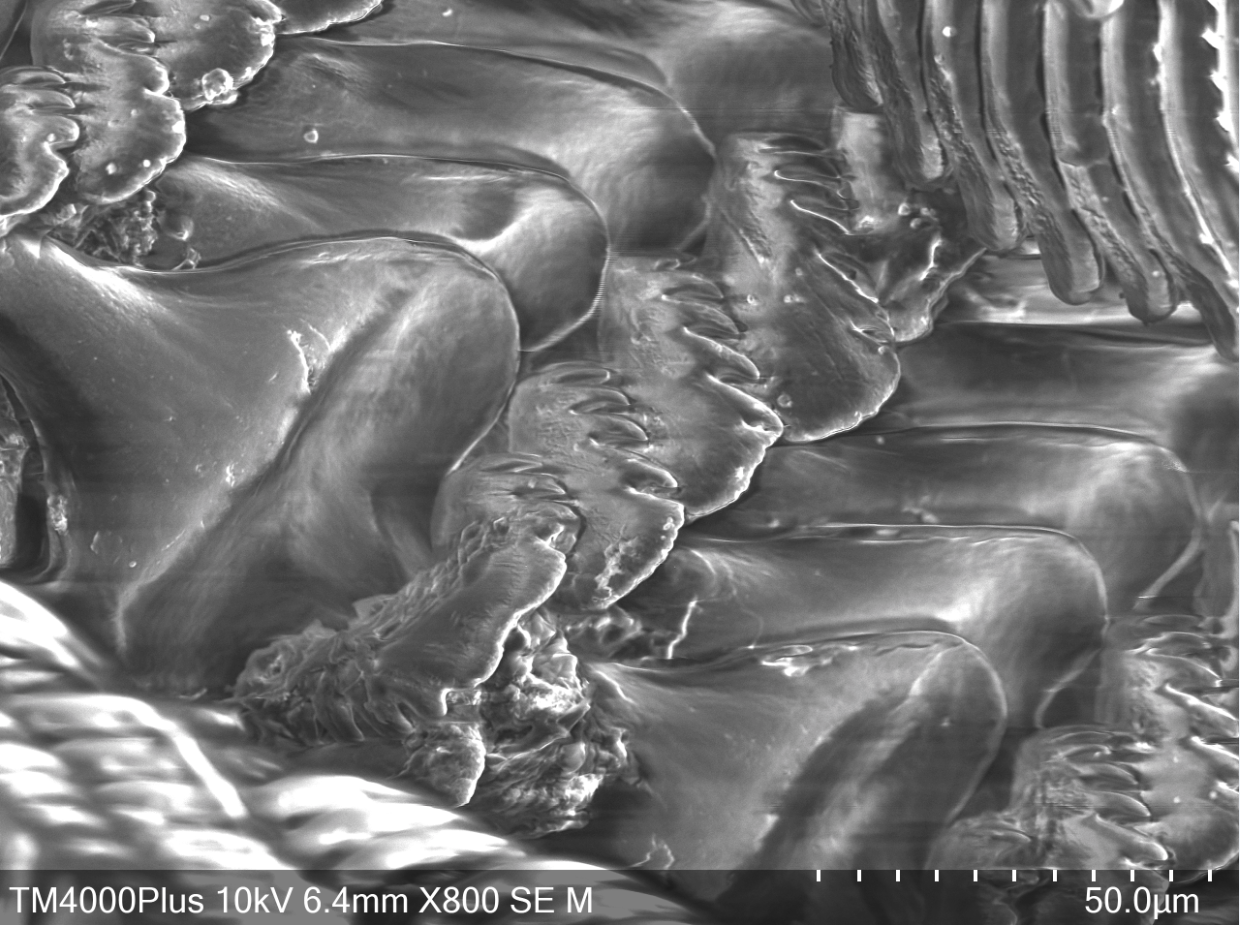
Figure 23: Scanning Electron Microscope image of A. porcata radula. Close-up of median and lateral teeth.
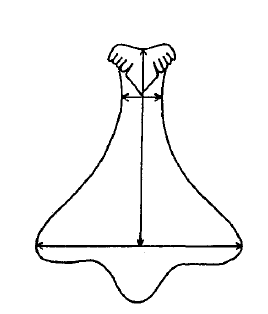
Figure 25: Diagram of median/rachidian tooth or A. porcata. Sourced from Parsons & Ward (1994), "Electrophoretic and Morphological Examination of Austrocochlea constricta(Gastropoda : Trochidae): A Species Complex."
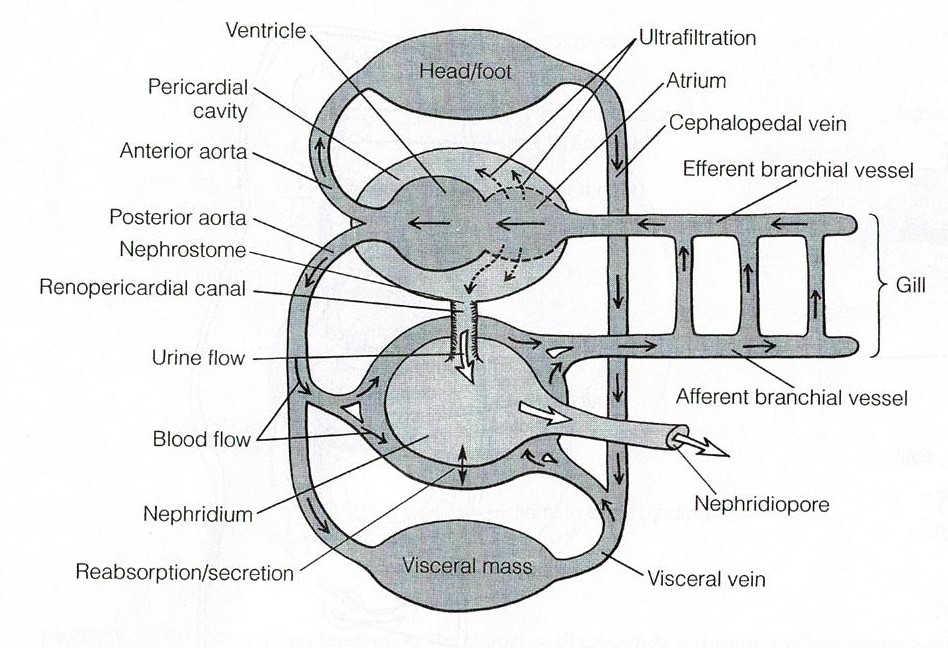
Hemal System
As with parts of the digestive system, torsion has moved
parts of the hemal system from posterior locations to anterior locations in the
visceral mass (Ruppert et al., 2004). This is what has occurred for the heart and
pericardial cavity (Figure 25). In most gastropods the right gill and right
atrium have been lost (Ruppert et al., 2004). The hemocoel has two major
components, the cephalopedal and visceral hemocoels (Ruppert et al., 2004). The
system is considered an open hemocoel system as the spaces are not lined by
epithelium (Ruppert et al., 2004). The ventricle usually receives oxygenated
blood via the atrium which is then pumped into the major vessels and aortae (Ruppert
et al., 2004). The visceral aorta supplies blood to the visceral hemocoel while
the cephalic aorta supplies blood to the cephalic hemocoel (Ruppert et al.,
2004). The aortae deliver blood to small vessels and large sinuses where
exchange with tissues is able to occur (Ruppert et al., 2004). Deoxygenated
blood passes over the nephridium and travels into the gills to become
oxygenated again, and subsequently exits to the atrium (Figure 25) (Ruppert et
al., 2004).
Back to top of page
|
 |
| Figure 20 |
|
 |
| Figure 21 |
|
 |
| Figure 22 |
|
 |
| Figure 23 |
|
 |
| Figure 24 |
|
 |
| Figure 25 |
|
|
|
|
Biogeographic Distribution | |
A. porcata is an endemic Australian species and has one
of the largest geographical distributions of its Austrocochlea relatives, extending much further north (Parsons
& Ward, 1994). Underwood (1974) characterised A. constricta’s distribution
as ranging from southern Tasmania (latitude 43°30'S.) to the tropics of
Queensland (23°S.). However, as this paper was written before A. constricta was split into 3 different
species (see taxonomy), it is likely that this distribution refers to A. porcata, especially when compared to
Parsons & Ward (1994), who found A.
porcata had a similar distribution to “A.
constricta” of Underwood’s work but who also found A. consticta did not range this far north (Figure 26).
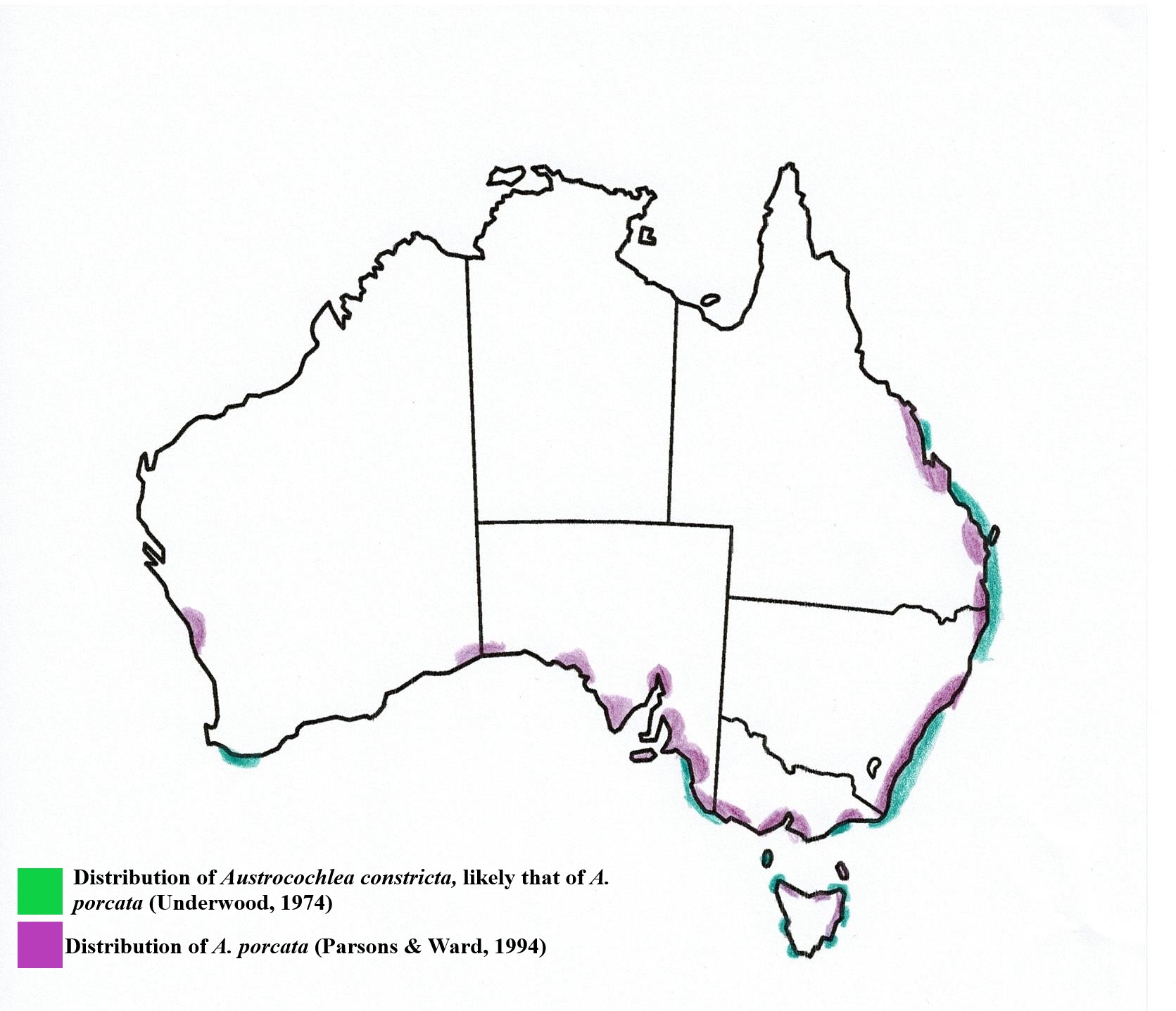
Figure 26: Geographic distribution of A.porcata
|
 |
| Figure 26 |
|
|
|
Evolution and Systematics | |
Figure 27: Taxonomy of Austrocochlea porcata.
The most current literature places Bivalvia as the sister
clade to Gastropoda (Kocot et al., 2011) This seems well supported with the use
of transcriptome and genome data from all of the major molluscan lineages (Kocot
et al., 2011). This places Gastropoda as the most derived molluscan group, thus
making synapomorphies such as torsion relatively recent innovations.
Within Vetigastropoda, the Trochoidea superfamily is more
derived than others within the group, such as Pleurotomarioidea (slit snails)
and Fissurelloidea (slit limpets) (Beesley, Ross & Wells, 1998). Trochoideans are characterised by
their conispiral shell that lacks a slit like their relatives (Beesley, Ross
& Wells, 1998). This shell type
reflects the loss of right ctenidium (gill structure) (Beesley, Ross &
Wells, 1998). Members of this
superfamiy have also lost their right ospharidium (olfactory organ).
However, within the Trochidae family itself, there seems to be
more uncertainty in regards to phylogenetic relationships between different genera,
and even more uncertainty within the Austrocochlea
genus. A. porcata has only
recently been considered separate from its sister species, A. constricta. Due to the variable nature of A. porcata’s banding patterns, A.
porcata was considered a polymorph of
A. constricta. Lidell (1950) was unable to find any anatomical differences
between morphs with different shell patterns or shape variations. Work by Creese & Underwood (1976) also found that A. constricta shell colouration may not
be genetically determined. Instead, it was suggested that the amount of
uroporphyrin pigmentation in the shell of different variants was highly
correlated with the concentration of chlorophylls in the substratum, an
environmental factor and not a genetic one (Creese & Underwood, 1976). This
particular study seemed to have actually used A. porcata specimens based on photos included in the manuscript,
which seems to be common in early literature. The classifying of A. porcata into the A. constricta species has lead to confusion regarding which
literature refers to each species.
Parsons & Ward (1994) investigated the troublesome A. constricta species complex using both
morphological characteristics and electrophoretic genetic analyses, using three
morphs found in Tasmania. Their results indicated that the 3 morphs were in
fact 3 different species; A. constricta, A. porcata and
A. brevis, with two
diagnostic loci separated A. constricta
and A. brevis (PEP-A and AAT-2), two
separated A. porcata and A. brevis (PEP-A and AAT-2), and one
separated A. porcata and A. constricta (PEP-A) in sympatric populations.
This finding was the first research to separate the species on a genetic basis
and indicates that A. porcata and A. constricta may be more closely related
than other Austrocochlean species.
The separation of A.
porcata and A. constricta into separate
species was supported by Donald, Kennedy & Spencer (2005). This study aimed
to resolve the relationships within the Trochidae family and in doing so
analysed the relationships between five Austrocochlea
species. Two mitochondrial genes and one nuclear gene were analysed, and both
the weighted maximum parsimony and Bayesian analysis
were used to construct phylograms of the family (Donald et al., 2005). These
phylograms supported Parsons & Ward’s (1994) results by separating A. constricta, A. porcata and A. brevis into three species (Figure 28).
It was expected that A. porcata and A. constricta would have the closest
genetic relationship based on the difficulty to separate them morphologically
(Lidell, 1950). However, with the inclusion of two more Austrocochlean species; A.
rudis and A. diminuta, a surprising
result was found. A. constricta and A. rudis were more closely related than A. porcata and A. constricta (Figure 28). This
is surprising given that these two species are morphologically distinct from
each other (Donald et al., 2005). In the context of the relationships between
genera, it was also found that the genus Australian genus, Chlorodiloma (resurrected from synonymy with Austrocochlea),
was more basal than Austrocochlea.
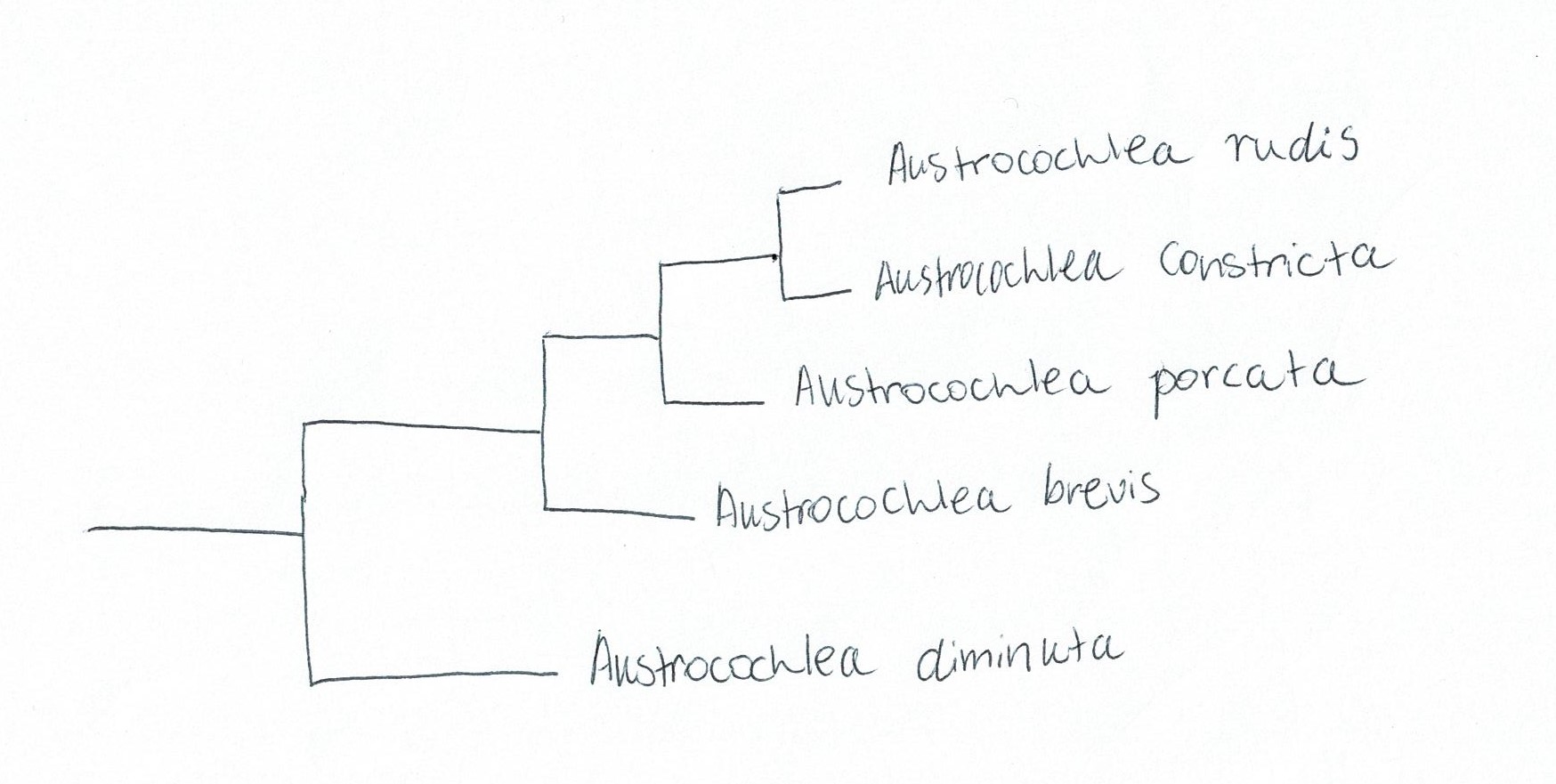
Figure 28: Simplified phylogram based off of maximum parsimony and Bayesian analysis of genetic data from the Austrocochlea genus. Adapted from Donald, Kennedy & Spencer (2005), "The phylogeny and taxonomy of austral monodontine topshells (Mollusca: Gastropoda: Trochidae), inferred from DNA sequences"
However, a later
study by Colgan & Schreiter (2010) had different
results. Colgan & Schreiter (2010) studied aimed to determine if the porcata or constricta phenotypes were genetically distinguishable, using both
mitochondrial DNA and nuclear gene intron DNA. The results however were not as definitive
as Paraons & Ward (1994) or Donald et al. (2005). Colgan
& Schreiter (2010) found that similar morphotypes did not form major
clades, or even subclades. Their maximum parsimony analyses of COI data found
three major clades: A. brevis, an
eastern clade and a western clade (Colgan & Schreiter, 2010). Both the porcata and constricta morphotypes were found in the eastern genetic clade but were
not found to form monophyletic sub-clades within this (Colgan & Schreiter, 2010).
Similarly, Parsons (1996) found that A.
constricta individuals from Western Australia possessed porcata-like shell banding patterns, but
these individuals were genetically more similar to constricta form individuals from Tasmania than to porcata form
individuals. Colgan & Schreiter (2010) suggest it could be possible that constricta/porcata phenotypes actually contain
four different species, but as of yet, the definite number of species contained
in the group is uncertain. However, in a nomenclatural sense, A. porcata is widely accepted as a separate
specied from A. constricta.
Synonyms:
Sourced from Parsons & Ward (1994).
-Trochus obtusus of authors (non Dillwyn).
-Monodonta zebra Menke, 1829: p. 17 (not Monodonta zebra Wood, 1828); Pilsbry, 1889: p. 91, pl. 20, fig. 20,
var.porcata, p. 92, pl. 20, figs. 10
and 11.
-Trochus constrictus: Quoy and Gairnard, 1834 (in part): p. 251, pl.
63, figs. 23 and 24; Philippi, 1846 (in part): p. 159, pl. 26, fig. 2a.
-Trochus taeniatus Quoy and Gaimard, 1834: p. 249, pl. 63, figs.
15-17 (not Trochus taeniatus Wood, 1828).
-Trochus zebra: Philippi, 1846: p. 160, pi. 26, fig. 4; Fischer,
1876: pp. 182-4, pl. 60, fig. 2; Smith, 1884: p. 74.
-Labio taeniata: A. Adams, 1851: p. 178.
-Labio porcata A. Adams, 1851: p. 179 (syntypes: British Museum
Natural History 196876 (3); loc.: Australia; coll.: H. Cuming; Fig. 5e).
-Trochocochlea multicarinata Chenu, 1859: 2, p. 360, fig. 2676;
Angas, 1867: p. 216.
-Trochocochlea taeniata: Chenu, 1859: p. 360, fig. 2677; Angas,
1867: p. 182; Tenison-Woods, 1877a (in part): pp. 89-96;
Tenison-Woods, 18776 (in part): p. 43.
-Trochocochlea porcata: Angas, 1867: p. 216.
-Trochus extenuatus Fischer, 1876: p. 330, pl. 103, fig. 1.
-Trochus multicarinatus: Fischer, 1876: p. 184, pl. 60, fig. 3.
-Trochocochlea zebra: Whitelegge, 1889: p. 270.
-Trochocochlea extenuata: Whitelegge, 1889: p. 270.
-Monodonta constricta: Tate and May, 1901 (in part): p. 405.
-Austrocochlea constricta: Pritchard and Gatliff, 1902 (in part):
pp. 123-4; Macpherson, 1961 (in part): pp. 173-6; Macpherson and Gabriel, 1962
(in part): p. 69; Macpherson, 1966 (in part): p. 211.
|
 |
| Figure 27 |
|
 |
| Figure 28 |
|
|
|
Conservation and Threats | |
Global warming poses a serious threat to many of the ocean’s
calcifying species. It is known that with an increase in the amount of carbon
dioxide (CO2) held in the ocean as a result of increased anthropogenic
activity, there is a great change in water chemistry (Turley, 2008). Not only
does the ocean’s pH decrease and become more acidic, but both the aragonite and
calcite saturation has drastically decreased (Royal Society, 2005). This is of particular
importance to species that use both aragonite and calcite in the formation of
their shells and skeletons (Kleypas et al., 2006; Lischka et al. 2011). A. porcata is one of these species and
requires carbonite minerals for the production of their shell (Coleman, Byrne
& Davis, 2014). Coleman et al. (2014) have demonstrated that under a
near-future ocean acidification scenario (increased water pH), A.porcata exhibits deteriorated shell
repair rate, shell structural integrity and snail condition. Decreased shell
repair rate and structural integrity were attributed to compromised
calcification ability under the near-future ocean condition scenario (Coleman
et al., 2014), while the loss in snail condition may have been a consequence of
increased metabolic cost when pH was reduced (Coleman et al., 2014). These
results demonstrate the likely dire outcomes for vulnerable species like A. porcata if ocean acidification
continues to rise with increased atmospheric CO2 levels. Furthermore,
it is likely that as a result of this decreased fitness, A. porcata will experience increased predation and competition from
less vulnerable species, potentially creating predator-prey relationships that
could cascade throughout intertidal communities (Coleman et al., 2014).
A. porcata is also
in danger from increased pollution in the marine intertidal zone. Cigarette
butts often find their way into the marine environments of coastal areas
(Booth, Gribben & Parkinson, 2015). Like other gastropods, A. porcata has been shown to be susceptible
to the effects of but leachate, with 100% mortality at full leachate concentration
of 5 butts per litre of water (Booth et al., 2015). Even at lower doses of 10%
and 25% leachate concentration, A.
porcata showed reduced activity when compared to other species and also had the greatest mortality out of the
species studied (Booth et al., 2015). These results clearly demonstrate the
vulnerability of A. porcata to increased
anthropogenic pollution.
|
|
|
References | |
Beesley, P.L., Ross, G.J.B. & Wells. A. (ed.) (1998). Mollusca:
The Southern Synthesis. Parts A and B: Fauna of Australia, Volume 5. CSIRO
Publishing, Collingwood Australia.
Booth, D. J, Gribben, P. & Parkinson, K. (2015). Impact
of cigarette butt leachate on tidepool snails. Marine Pollution Bulletin, 95, 362-364.
Brusca, R.C. & Brusca, G.J. (2003). Invertebrates
(Second Edition). Sinauer Associates Inc., Sunderland, Massachusetts.
Brusca, R.C, Moore, W. & Shuster, S.M. (2016).
Invertebrates (Third Edition). Sinauer Associates Inc., Sunderland, Massachusetts.
Chilton, N.B & Bull, C.M. (1984). Influence of predation
by a crab on the distribution of the size groups of three intertidal gastropods
in Australia. Marine Biology, 83,
163−169.
Coleman, D.W., Byrne, M. & Davis, A.D. (2014). Molluscs
on acid: gastropod shell repair and strength in acidifying oceans. Marine Ecology Progress Series, 509, 203–211.
Creese, R.G. & Underwood, A.J. (1976). Observations on
the biology of the trochid gastropod Austrocochlea
constricta (Lamarck) (Prosobranchia). I. Factors affecting shell-banding
pattern. Journal of Experimental Marine
Biology and Ecology, 23, 211–228.
Donald, K.M., Kennedy, M. & Spencer, H.G. (2005). The
phylogeny and taxonomy of austral monodontine topshells (Mollusca: Gastropoda:
Trochidae), inferred from DNA sequences. Molecular
Phylogenetics and Evolution, 37,
474–483.
Fairweather, P G., Underwood, A. J. & Moran, M. J.
(1984). Preliminary investigations of predation by the whelk Morula marginalba.Marine Ecology
Progress Series, 17, 143-156.
Giese, A. C. (1959). Comparative physiology: Annual
reproductive cycles of marine invertebrates.
Annual review of physiology,
21, 547-76.
Kleypas, J.A., Feely, R.A., Fabry, V.J., Langdon, C. &
Sabine, C.L. (2005). Impacts of ocean acidification on coral reefs and other
marine calcifiers: a guide to future research. Report of a workshop held 18−20
April 2005, St. Petersburg, FL, sponsored by NSF, NOAA, and the US Geological
Survey. Contribution No. 2897, NOAA/Pacific Marine Environmental Laboratory,
Seattle, WA.
Liddell, J. (1950). An examination of the genus Austrocochlea
Fischer. M.Sc. Thesis, University of Sydney, Sydney.
Lischka, S., Budenbender, J., Boxhammer, T. & Riebesell,
U. (2011). Impact of ocean acidification and elevated temperatures on early
juveniles of polar shelled pteropod Limacina
helicina: mortality, shell degradation, and shell growth. Biogeosciences, 8: 919−932.
MacPherson, J.H. & Gabriel, C.J. (1962). Marine molluscs
of Victoria. Melbourne University Press, Melbourne.
Mapstone, B. D., Underwood, A.J. & Creese, R.G. (1984). Experimental analyses of the commensal
relation between intertidal gastropods Patelloida
mufria and the trochid Austrocochlea
constricta. Marine Ecology Progress Series, 17, 85-100.
Miller, S.L. (1974). Adaptive design of locomotion and foot
form in prosobranch gastropods. Journal
of Experimental Marine Biology and Ecology, 14, 99-156.
Parsons, K.E. (1996). Discordant patterns of morphological
and genetic divergence in the ‘Austrocochlea
constricta’ (Gastropoda: Trochidae) species complex. Marine and Freshwater Research, 47, 981–990.
Parsons, K.E. & Ward, R.D. (1994). Electrophoretic and
morphological examination of
Austrocochlea constricta (Gastropoda: Trochidae): A species complex. Australian Journal of Marine and Freshwater Research,
45, 1065–1085.
Pechenick, J. A. (2010). Biology of the Invertebrates (Sixth
Edition). Higher Education, McGraw Hill Companies.
Royal Society (2005). Ocean acidification due to increasing
atmospheric carbon dioxide. Policy document 12/05 Royal Society, London. The
Clyvedon Press Ltd, Cardiff.
Ruppert, E.E., Fox, R.S. & Barnes (2004). Invertebrate
Zoology: A Functional Evolutionary Approach (Seventh Edition). Brooks/Cole
Publishing.
Turley, C. (2008). Impacts of changing ocean chemistry in a high-CO2
world. Mineralogical Magazine, 72, 359–362.
Underwood, A. J. (1972). Spawning, larval development and
settlement behaviour of Gibbula cineraria
(Gastropoda: Prosobranchia) with a reappraisal of torsion in gastropods. Marine Biology, 17, 341-349.
Underwood, A.J. (1974). The
reproductive cycles and geographical distribution of some common Australian
prosobranchs (Mollusca: Gastropoda). Australian
Journal of Marine and Freshwater Research, 25, 63–88.
|
|
|
|
|