Life History & Behaviour
Locomotion
Holothuria hilla are slow and very sluggish animals. Only three of out five rows of tube feet are well developed and used to move on substrates (Figure 3a, 3b). When dislodged from substratum, the animal twists its anterior end until the tube feet is in contact with substrates again (Ruppert et al 2004).

Figure 3a. Picture displaying H. hilla's tube feet on substrates.

Figure 3b. Picture exhibiting the adhesiveness of H. hilla's tube feet.
Reproduction
H. hilla propagates primarily by sexual reproduction (Mackey 2001). Males and females are usually separate individuals. Sexual reproduction in H. hilla comprises of gametogenesis and spawning phases. Gametogenesis is the process of developing sperm and ova which occurs in the animal’s single gonad. Spawning takes place when mature gametes are released into the water column.
H. hilla also reproduce via asexual reproduction through fission (Lee et al 2008). The animal splits into two equal parts by constricting its body in the middle region (Mackey 2001) (Figure 3c). After twisting continuously at the constriction site, the body wall eventually ruptures. The bisected parts regenerate internal organs separately to result in two individuals. Individuals that have undergone fission are easily identified by the lighter colour and smaller regenerating region (Lee et al 2008) (Figure 3d).
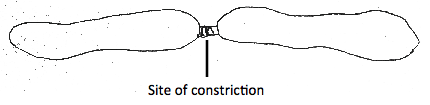
Figure 3c. Simplified diagram showing an asexual reproduction method by H. hilla by twisting forcefully, thus constricting the middle of its body. (Adapted from: Mackey 2001)

Figure 3d. Picture showing results of asexual reproduction (fission) of H. hilla. Black arrow indicate regenerating region. (Adapted from: Lee et al 2008)
Respiration
Both tube feet and buccal podia function as gills but the main gas-exchange organ of H. hilla is a pair of internal respiratory trees (Ruppert et al 2004). Ventilation of respiratory trees is aided by muscular pumping of the cloaca (refer to the short clip below). Several short inhalations by the cloaca are needed to fill the respiratory trees, after which, all of the water will be expelled in one prolonged exhalation.
Feeding
Introduction & Aims
Like most bottom-dwelling sea cucumbers, H. hilla feeds on algae, bacteria, microorganisms and organic detritus that are present in sediments (Preston 1993; Goemans 2012; Foster & Smith 2012). Feeding takes place when the organism creeps over substratum with the mouth facing the bottom (Preston 1993). It fully extends the tentacles and uses its mucous lined buccal podia to adhere sediments. Thereafter, each tentacle shoves sand into the mouth with the sphincter muscles regularly closing and opening. Past studies have indicated that bottom-dwelling holothurians hold a paramount role in recycling organic detritus in benthic ecosystem (Preston 1993; Shakouri et al 2009; Afkhami et al 2012).
A study was conducted in the Heron Island Research Station (HIRS) to analyse the extraction efficiency of organic substances from sediments consumed by H. hilla. It is hypothesized that there will be significantly lesser organic materials in the fecal pellets than in the sediments before ingestion by H. hilla.
Materials and Methods
Figure 3e. Map showing the collection site for H. hilla.
Six specimens of H. hilla were collected from Shark Bay and Southern Reef Flat on Heron Island during low tide in the late afternoon (Figure 3e) When a specimen was spotted, labeled 50mL FalconTM tubes were used to collect sediments at the anterior end of the animal. The animal was then placed in labeled ziplock bags and brought back to HIRS. Seawater within the bag was consistently changed every 15 minutes on the field to ensure constant supply of oxygen for the animal. Specimens were kept in aquariums with 24 hours constant fresh sea water supply at the research station (Figure 3f).

Figure 3f. An example of how the specimens are kept at HIRS.
Treatment for sediments at anterior end that were brought back to HIRS
- Sediments were placed in labeled ceramic bowls.
- These bowls were then placed in the drying cupboard at 70°C overnight.
- The sediment-filled bowls were removed from the drying cupboard, weighed and the mass was recorded.
- The bowls were then left in a muffle furnace at 525°C for 3 hours to burn off all organic materials.
- The bowls were set aside to cool before they were weighed individually and recorded again.
Fecal pellets were collected from each of the specimens’ tanks and placed in labeled ceramic bowls. Steps 1 – 5 were repeated for fecal pellets collected and data was logged.
Results & Discussion
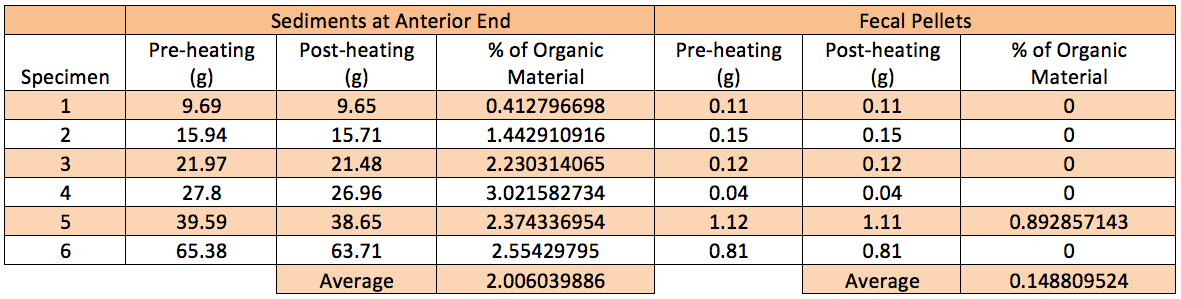
Table 1. Collated data of weight of sediments at anterior end and fecal pellets before and after heating. Percentage of organic material was calculated as shown.
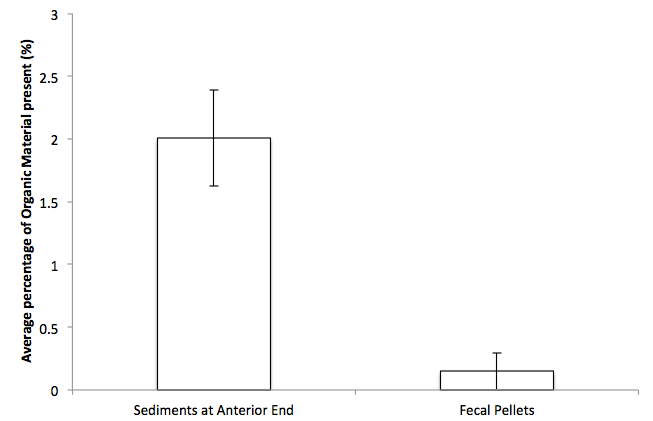
Figure 3g. Column graph displaying the average percentage of organic material present in both sediments at anterior end and fecal pellets. Error bars represent ± SEM.
Data collected showed that almost all of the organic materials that were present in the sediments removed (Table 1 & Figure 3g). Thus, organic materials are significantly lesser in the fecal pellets than sediments at anterior ends of specimens (p<0.05). This indicates that H. hilla has a gut system that is highly efficient in extracting organic materials from sediments. This will be highly advantageous for the animal that lives in an environment that has substratum with low percentage of organic sediments.
Further studies can be done with more specimens and perform dissections to look further into the anatomy of the digestive system of H. hilla.
|