Distribution
Chaetognaths are an important component of the zooplankton community, and are found in all marine waters, occupying both benthic and planktonic habitats.
Africa:
Studies along the west coast of Africa have identified numerous different species of Chaetognatha. On two sample expeditions off the coast of Angola (Neto 1961), 18 species were identified, with Sagitta friderici, a neritic species, being the most abundant, accounting for 22.6 and 23% of all species. Whilst in a sample expedition along the coast of Congo (Furnestin 1962, Gibbons 1992), S. friderici was again the most abundant species, comprising 45.7% of all chaetognaths sampled (Furnestin 1962). However, other studies along the same coasts have identified different species compositions (Ducret 1968), with S. enflata and S. hexaptera been the most dominant (49.8 & 20.5%, respectively). The proposed reasoning for this difference was that the samples were collected over waters beyond the continental shelf (>1000m), whilst S. friderici is believed to be a coastal species. This is the same case identified off Argentina, as S. enflata’s abundance decreased towards the coast, and S. friderici’s increased (Almeid-Prado 1968). There have also been a large number of chaetognaths identified off the Namibian coast (Duro & Gili 1996). Duro & Gili (1996) also identified a fine-scale relationship between species and the Benguela current, with S. setosa accounting for 70% of species found in cold up-welled waters.
South America:
A study off Brazil identified a seasonal difference in abundance and species composition, due to the influence of tropical waters in summer, and Antarctic waters in winter (Resgalla 1993). This study found 13 species common to both seasons, with 3 (S. gazellae, S. maxima and Eukrohnia bathypelagica) that were unique during winter, and 2 (Krohnitta pacifica and S. bipunctata) that were unique to summer. There are currently 39 chaetognath species identified in the South Atlantic, with the biodiversity of chaetognaths in the Atlantic one-third lower off the coast of South America (26 species), compared to Africa (38 species).
Water currents may be the main factor affecting the distribution of species. Although the Agulhas current is only small, it greatly affects the biodiversity of chaetognaths on the west coast of Africa (figure 1). Whilst the south bound Brazil current, and northbound Malvinas/Falkland current to the west, the Benguela current to the east, and the Subtropical and Antarctic Convergence zones are the main factors affecting the distribution of species.
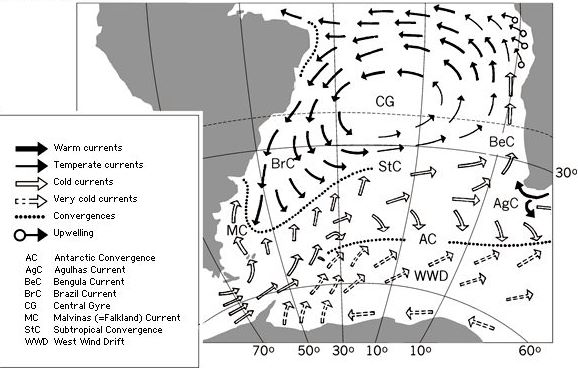
Figure 1: Surface currents of the south Atlantic. From MSIP.
Other:
There has even been a fully troglobitic (cave dwelling) species (Paraspadella anops) that has only been found in one cave in the Bahamas (Casanova 2011). This species has developed special adaptations that allow it to thrive in this environment, including the loss of eyes and no body pigmentation.
Deep-Sea:
There are two deep-living (>700m) species (Eukrohnia fowleri and Caecosagitta macrocephala) that have been identified as containing bioluminescent organs (Thuesen et al. 2010). Bioluminescence is used by many marine organisms, for both offensive and defensive actions, as well as within species for mate attraction. Despite C. macrocephala been a common deep-living species, it took 90 years of studying it before bioluminescence was described for it. It has been suggested that one possible reason for this delay was that the light organs were getting damaged when the specimens were been collected during plankton tows. Studies into the production of bioluminescence have shown that this emitted light is produced by specialized photocytes that react luminous materials, including luciferin and luciferase. The placement of these organs differs between these two species; in C. macrocephala they are located ventrally on the posterior two-thirds of the anterior fin, whilst, in E. fowleri, the bioluminescent organs are located on both the ventral and dorsal sides of the tail fin (figure 2). It has also been shown that these two species release bioluminescent clouds when startled, this is believed that the purpose of this is to act as a distraction, to confuse and ward off predators.
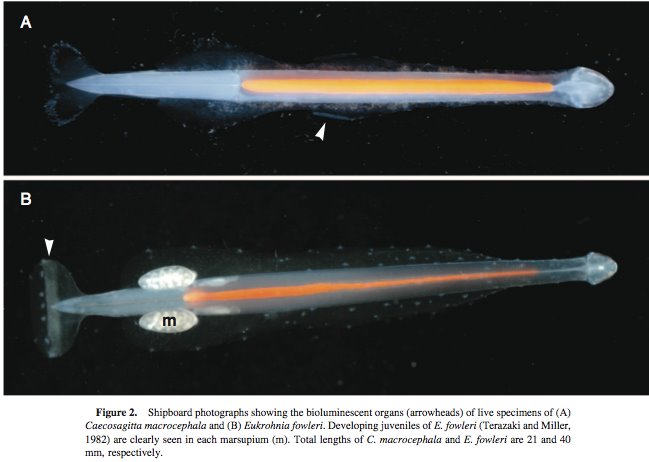
Figure 2: Bioluminescent organs of C. macrocephala & E. fowleri. From Thuesen et al. 2010
|