Behaviour
Feeding:
Chaetognaths are all carnivorous predators of copepods, fish
larvae and other zooplankton (Gibbons 1992, Ruppert et al. 2004). They are sit-and-wait
predators in the water column that detect their prey using their ciliary tufts
(Foster 2006), and once detected, they retract their hood and lunge toward
their prey and grab it using their grasping spines (video 1). It has been
identified that some chaetognaths inject captured prey with a neurotoxin,
Tetrodotoxin, which assists by immobilising they prey, and as chaetognaths’
bodies are translucent; this makes it possible to view what they have just
eaten. (Thuesen et al. 1988, Thuesen & Kogure 1989, Foster 2006). The maximum
distance for detection of prey has been calculated for three species, Spadella
schizoptera and Sagitta hispida cannot detect prey >3mm away, whilst Sagitta
elegans has a maximum detection of between 0.09 and 13.2mm depending on the
prey species (Foster 2006). These results also suggest that there may be a
minimum detectable size of prey, and speed at which the prey must be traveling
at.
Swimming:
Chaetognaths swim in a dorsoventrally undulating sink-swim
pattern, by contracting their longitudinal muscles on their trunk, causing the
head to bend backwards, and then flick themselves forward with their caudal fin
(Ruppert et al. 2004, Foster 2006, Casenove 2011). When chaetognaths are in
their ‘sink’ stage, they use their posterior fins to stabiliser themselves, and
prevent themselves from sink too much.
Video of a Chaetognath capturing a prey. Courtesy of Taichiro Goto.
Vertical migration:
Vertical migration in chaetognaths in most prominent in offshore species, than it is for inshore species (Gibbons 1992). It is believed that the main reason for this migration in offshore species is that chaetognaths are using their mechanoreceptors to follow their prey (other planktonic organisms) as they undergo diel vertical migration (Alvarino 1964, Gibbons 1992). It has also been suggested that the possible reason why inshore species do not display a strong vertical migration, may be because if increased water mixing from the different strata as they are transported onshore, and also due to hydrological turbulence (Gibbons 1992). Although it may seem that chaetognaths are just following their prey as they undergo these diel vertical migrations, studies have shown that chaetognaths do possess photo-tactic abilities (Goto et al. 1984, Foster 2006, Thuesen et al. 2010). The photo-tactic organ of chaetognaths is their eye, which is composed of a single centrally-situated pigment cell with photoreceptive cells around it (Goto 1984, Foster 2006).
Migration/Phototaxis Experiment:
Methods: To test the photoreceptive ability of chaetognaths, 18 plankton dips were performed off the jetty (three in the afternoon (3pm), and three at night (7pm)), and six 5-minute plankton tows were performed off the western side of Heron Island, Australia (at 2pm), over three days. The plankton dip was performed by dropping a dip net to the benthos, allowing the area to settle for a few minutes, and retracting the net back to the surface over a 3-minute period. The plankton tow was performed by towing a plankton net behind a boat for 5-minutes at 3 knots, just off the reef crest. The plankton samples were then analysed for their species composition, and all chaetognaths were separated from the rest of the specimens.
Methods/Materials: To evaluate the degree of photoreceptiveness, a chaetognath was placed into a 30cm long plastic tube (diameter = 10mm), which was mounted between two tripods (figure 1). Two cardboard partitions, with a hole in the middle, were then placed at 5 & 20cm from a light source. The light was turned on (figure 2), and the contraption was covered in a black drop-sheet to eliminate any external light, each trial was run for approximately 7 minutes. The light was then turned off, and a light on the opposite side was then turned on, to investigate if the chaetognath migrated to the other side. There were a total of eight trials performed: the second and used the Yahata model, whilst all other trials used the Yahata light. Two of these trials were run with green-cellophane (figure 3), and two with red-cellophane (figure 4) covering the light, this made it possible to determine if the chaetognaths were able to detect red or green light.
The lights used were: Yahata (Model: LG-PS2-5, Olympus Corporation, fibre optic light), and an Olympus SZS1 (12 volt).
Results/Discussion: There were some problems encountered with the experiment, namely that once the chaetognaths were placed in the plastic tube, they died after the first or second trial.
There was a light intensity difference observed between the two lights, with the Yahata emitting a more intense light, and the Olympus a less intense. Comparing these two lights identified that with the less intense light, chaetognaths approached closer to the source (i.e. between the light and the first partition), whilst chaetognaths subjected to the more intense light only advanced as far forward as the first partition, and did not go beyond it.
This result supports those that have identified that chaetognaths have phototaxis abilities (Goto et al. 1984, Foster 2006, Thuesen et al. 2010). This differentiation between light intensity may assist the chaetognaths in ensuring they do not swim to close to the surface, whilst also ensuring they can follow the same vertical migration pattern of other zooplankton, when they are too far away to be detected with their ciliary tufts.
Additionally, when using the red-light, the chaetognaths did not show any reaction, whilst when subjected to the green light, they had a positive reaction and moved towards the light. This result conforms to general knowledge that red light is absorbed more rapidly than green light; therefore it is uncommon for most marine species to have red light receptors, and you would not expect to see a reaction to red light.
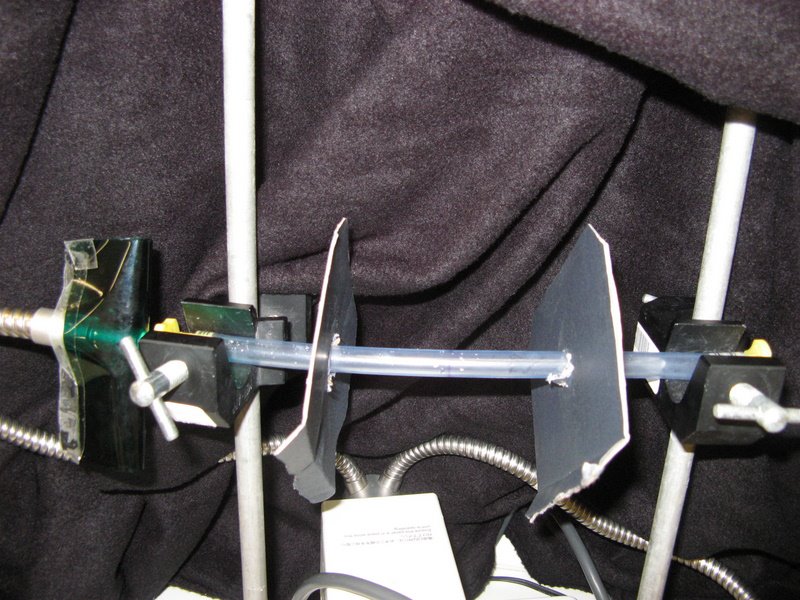
Figure 1: Experimental set-up for photoreceptiveness.
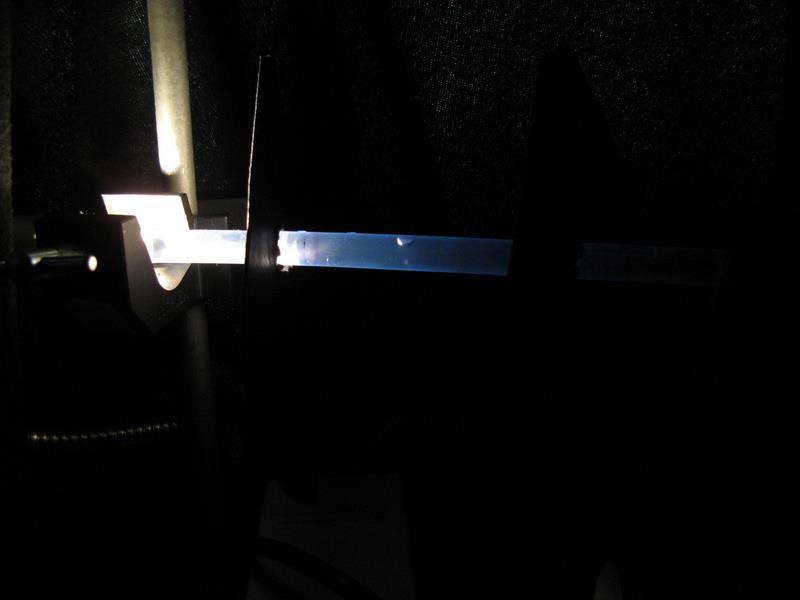
Figure 2: Experimental set-up for white-light photoreceptiveness.

Figure 3: Experimental set-up for green-light photoreceptiveness.

Figure 4: Experimental set-up for red-light photoreceptiveness.
|